Metal sources and deposition through time
Applying a paleolimnological approach at Long Lake resulted in a 10 500 year record of metal deposition in lakebed sediments and indicated that lakebed sediment concentrations of Hg fluctuated substantially, and in some sections of the core exceeded the interim sediment probable effects level (PEL) established by the
CCME (1999) (
Table 3). XRF measurements reported Zn concentrations that were above the interim sediment quality guidelines (ISQG) and As concentrations that were above the PEL; however, the degree to which XRF measurements from this study are consistent with the values reported by the
CCME (1999) is unknown. The bulk geochemical data were effective in identifying that significant natural and anthropogenic environmental changes have occurred and have influenced both sediment composition and metal deposition.
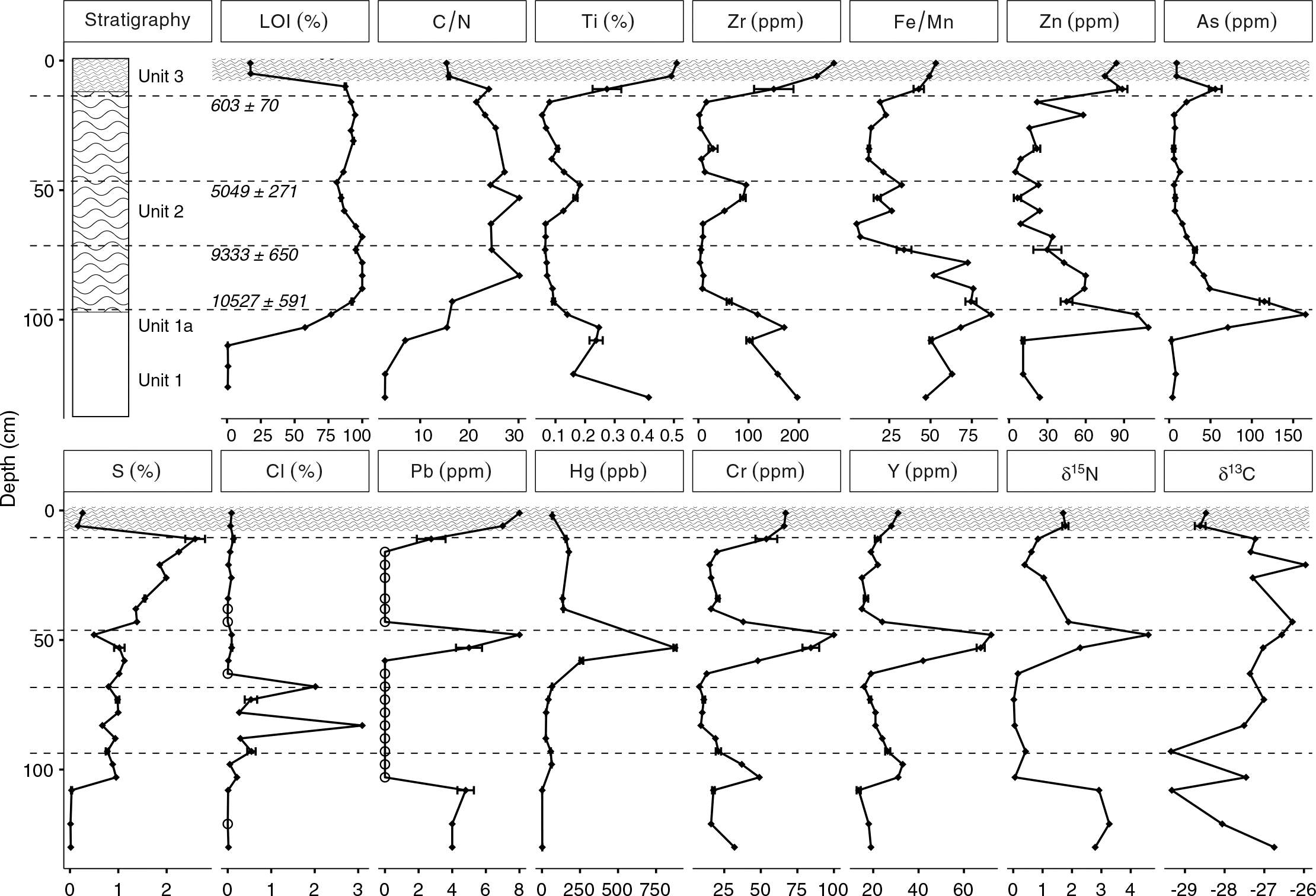
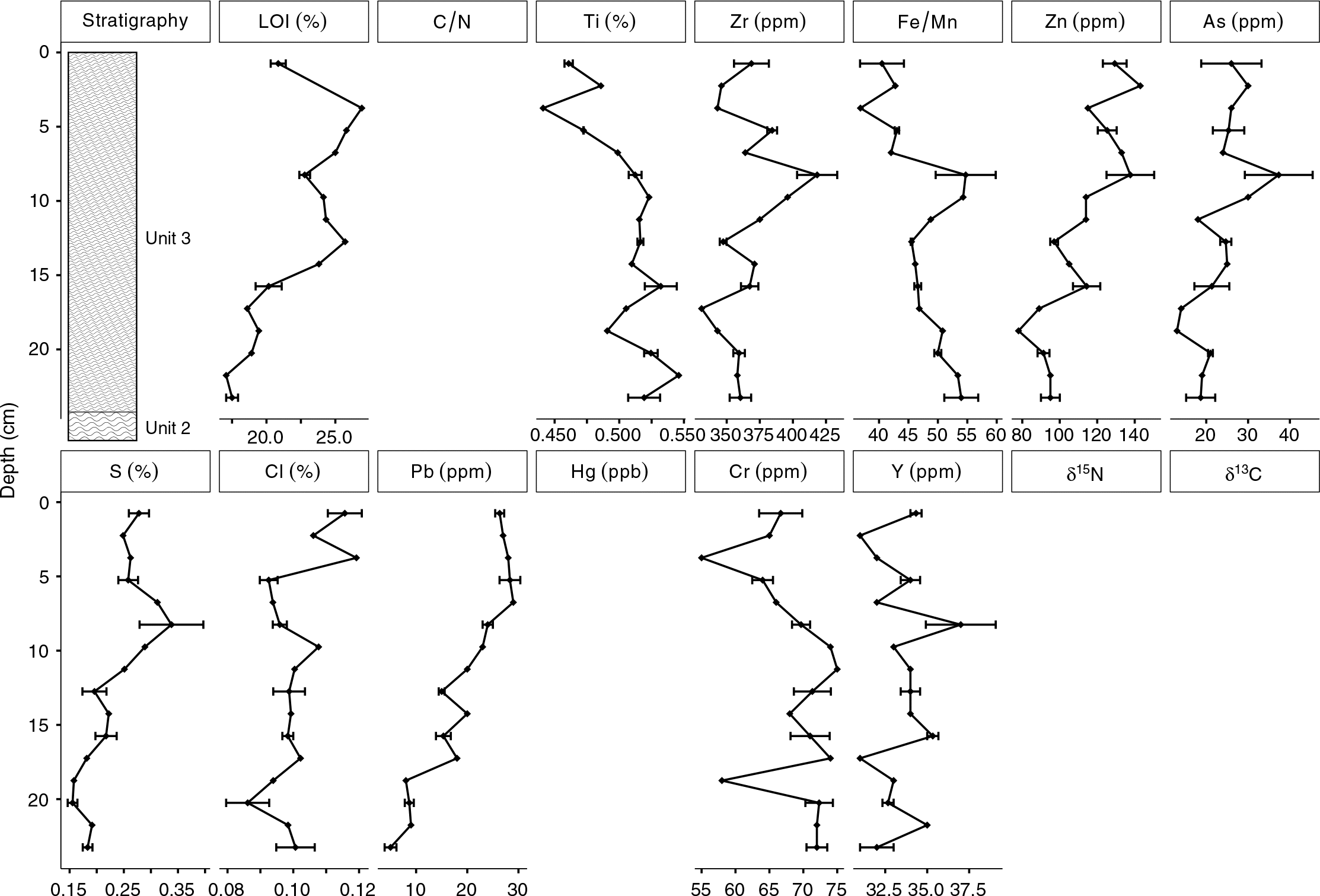
Lead values were highest in Unit 1 (∼4 ppm) and Unit 3 (∼20 ppm), both of which were likely minerogenic and were derived, in part, from till deposits that have a northwest provenance (
Stea 1982;
Foisy and Prichonnet 1991;
Shaw et al. 2002). The strataform Pb-Zn deposits in the underlying Boss Point Formation (
New Brunswick Department of Natural Resources and Energy 2002) are the likely source based on paleo-iceflow direction and the coincident elevated levels of Pb, Zn, and Ti in Unit 3 and Unit 1. Although lead shot is considered a significant source of bioavailable lead in the wetlands of Atlantic Canada (
Stevenson et al. 2005), in this study, evidence of its impact in our bulk geochemical analysis of lakebed sediments in the CMR was not observed. Although the CMR was subjected to significant waterfowl hunting pressure throughout the 19th and 20th centuries (
Schwab and Daury 1989), lead shot was banned in 1999 and the water chemistry at Long Lake (pH ∼7.0) is not conducive to lead mobility (
Outridge and Wang 2015). Other paleolimnological studies in Nova Scotia noted significantly higher Pb concentrations in lakebed sediments, associated primarily with local industrial and residential development (
Terry 2011;
Misiuk 2014).
Arsenic and Cr were variable but had elevated concentrations in the Long Lake sediments. The vertical distributions of As and Cr concentrations in the Long Lake sediments were likely affected by changes to the redox environment in the sediment or water column, as high sedimentary As and Cr concentrations have been reported at the onset of anoxia in some lakes (
Schaller et al. 1997). Multiple sources for these metals are likely and include minerogenic material, groundwater, and atmospheric deposition. The Taylor Village paleo-placer deposit, located 18 km from Long Lake, contains anomalous Cr, Ti, and Y; the bedrock source for the mineralization is likely the Coverdale Anorthosite Complex (
Hudgins 1999;
Barr et al. 2007). It is likely that these elements were transported to the Long Lake watershed in till. The copper and sulphur form organic (chelate) complexes (
Fraser 1961) and the source bedrock are thought to be the underlying Boss Point Formation. The pathway for these minerals may have been metal-rich groundwater ascending major permeable structures such as the nearby Dorchester Fault. The source of the metals may be the underlying sandstone, but this has not been demonstrated.
Arsenic and Zn concentrations were particularly high in Unit 1a (As >150 ppm and Zn >90 ppm). Similar to Cr, the vertical distribution of Zn concentrations in the Long Lake sediments was likely also affected by changes to sediment or water column chemistry (
Outridge and Wang 2015). The primary source was likely the mineral arsenopyrite (AsFeS
2) found in regional soils and till derived from a wide variety of bedrock types in both Nova Scotia and New Brunswick, and Pb-Zn deposits that were also a likely source of Pb to the Long Lake sediments (
New Brunswick Department of Natural Resources and Energy 2002).
Craw et al. (2003) showed that arsenopyrite is soluble in oxidizing conditions while being insoluble or stable in moderately reducing environments, suggesting that redox changes at the sediment–water interface may concentrate As in sediments that experience sudden changes in redox state (
Masscheleyn et al. 1991;
Schaller et al. 1997). Sources for As and Zn into Long Lake include the erosion and weathering of As- and Zn-bearing sediment, with elevated values at the top of Unit 1a and base of Unit 3 being a result of post-depositional mobility.
Increases in Pb, Hg, and δ
15N in the core at 5500 cal BP (
Fig. 3) were likely the consequence of regional fires and indicated that a significant reservoir of these metals exists in the natural environment. Increases in Hg have been observed following wildfires and associated storm runoff (
Garcia and Carignan 1999;
Caldwell et al. 2000), in addition to increases in δ
15N (
Spencer et al. 2003). The fire events were broadly coincident with a period of warming that may have also been a period of low precipitation in Atlantic Canada (
Mott et al. 2009;
Spooner et al. 2014). Wetter conditions and a raised water table at the site have persisted since 4000 cal BP, likely as a result of changing climate conditions and rapid salt marsh aggradation in response to sea level rise (
Shaw and Ceman 1999). Unlike other metals that peaked at this time, the concentrations of Hg following the event were elevated by a factor of 2 compared with concentrations prior to the event, suggesting the potential for Hg to remain elevated following its introduction to a system (
Rydberg et al. 2015).
Peak concentrations of As, Hg, and Zn all exceeded Canadian ISQG guidelines, and, in the case of As and Hg, exceeded the probable effects level as established by the
CCME (1999) (
Table 3). Peak concentrations of As, Hg, and Zn all occurred prior to European settlement of North America, suggesting the potential for guideline-exceeding concentrations of these elements to occur due to natural phenomena. Surficial concentrations of As and Zn also exceeded ISQG guidelines, likely as a result of increased erosion from human-induced water level change and atmospheric deposition.
Potential for sequestration and release
Surficial concentrations of metals in the Long Lake sediments were low, but much higher concentrations were found deeper in the core (i.e., from older dates). Elevated concentrations of As, Zn, Hg, Y, and Cr all were found at a depth of 50 cm, and it is unlikely that this horizon is unique to Long Lake. Natural resuspension at this depth is unlikely, but the engineering of wetlands in the Amherst Marsh is ongoing (
Dunnington 2011), and future resuspension due to human activities is likely. Suspending particles with elevated metal concentrations has the potential to increase the bioavailability of these metals, and should be avoided in the CMR given our results. Similarly, higher concentrations of metals in till surrounding Long Lake have the potential to be liberated with development or water level changes that may increase shoreline erosion (
Kalnejais et al. 2007).
Elevated concentrations of As and Zn were found at both transitions (Unit 1 to Unit 2; Unit 2 to Unit 3), suggesting that their deposition may have been related to environmental changes that occurred at these times. The solubility of As and Zn are affected by changes in redox chemistry at the sediment–water interface, particularly a decrease in effective solubility in oxidizing conditions, as As in particular is enriched in sediment by Fe and Mn precipitation (
Masscheleyn et al. 1991;
Schaller et al. 1997). When oxidizing conditions are present, more of these metals are removed from the water column and sequestered in sediments. Conversely, when reducing conditions are present, there is a potential for metals sequestered in sediment to be released (
Aggett and O’Brien 1985). The CMR is a shallow-water system, and large changes in redox are unlikely but possible in the case of algal blooms or encroachment of bog, both of which promote reducing conditions (
Cohen 2003).
Hg values in Unit 2 were elevated compared with the values measured in Unit 1, which is consistent with the process of available Hg readily complexing with organic matter (
Lepane et al. 2007;
O’Driscoll et al. 2011). However, the greatest concentration of Hg occurred during the peak of other metals ca. 5000 cal BP, coincident with a time of high clastic input to Long Lake. It is, therefore, likely that the sequestration of Hg in the Long Lake sediments was a result of increased supply to the water column with constant removal rates from the water column due to complexing with available organic matter.