Introduction
Temperature remains one of the most significant abiotic factors affecting the biology of fishes (
Fry 1958;
Hochachka and Somero 2002;
Currie and Schulte 2014) primarily because their body temperatures change concomitantly and rapidly with ambient temperatures (
Stevens and Sutterlin 1976). High water temperatures in the summer months have been implicated in the decline of the Atlantic salmon (
COSEWIC 2010) in Canada, one of the country’s most important commercial and recreational fishes. For example, the Miramichi River in New Brunswick, Canada, home to one of North America’s largest Atlantic salmon runs, is reaching exceptionally high water temperatures during the summer months (e.g., 27–30 °C;
Caissie et al. 2014), exceeding the 23 °C proposed upper tolerance limit of Atlantic salmon as often as 62 d/year (
Lund et al. 2002;
Caissie et al. 2012). Beyond this temperature, adult Atlantic salmon have been reported to cease feeding, abandon territories, and aggregate in cool water refugia (
Breau et al. 2007,
2011). Temperature changes are rapid, greater than 8 °C/d and up to 1.4 °C/h, allowing little acclimatization or recovery time (
Lund et al. 2002;
DFO 2012). It is important that we understand tolerance limits and the physiological changes underpinning temperatures representative of thermal events that Atlantic salmon would normally experience in the wild.
Most of our understanding regarding the effects of acute temperature change on salmonids comes from studies where fish are acclimated to one temperature and subjected to dramatic and rapid changes (i.e., over 1–2 h; e.g.,
DuBeau et al. 1998;
Currie et al. 2000;
Galloway and Kieffer 2003;
Fowler et al. 2009). Although such studies provide important mechanistic insight into the capacity of salmonids to cope with acute thermal stress, they do not readily allow extrapolation to natural conditions. Comparatively fewer studies examine acute thermal scenarios representative of field conditions (but see
Threader and Houston 1983;
Houston and Gingras-Bedard 1994;
Mesa et al. 2002;
Podrabsky and Somero 2004;
Todgham et al. 2006;
Narum et al. 2013;
Eldridge et al. 2015 for fish examples). Recently,
Tunnah et al. (2017) compared physiological responses to distinct, multiday natural diel thermal cycling scenarios in wild Atlantic salmon and found no obvious differences, concluding that cycling itself is more important than the nature of the thermal cycle (
Tunnah et al. 2017). In contrast, in rainbow trout metabolic reserves are depleted in a single diel thermal cycle (16–24 °C) but recover with multiple days of cycling (
Callaghan et al. 2016). One outstanding question from this collective work was the immediate physiological effects of a short-term, environmentally relevant, acute heat shock, and whether or not this exposure could influence tolerance to a later, more severe thermal stress. If we hope to forecast impacts of climate warming on fishes, we need early temporal resolution to assess physiological and cellular changes in environmentally realistic contexts.
Our first goal was to describe the responses to a single heat challenge in the lab representative of field conditions, in an effort to understand the underlying physiological responses. To this end, we exposed hatchery-reared Atlantic salmon to thermal conditions representative of a well-known Atlantic salmon-producing river. We measured key metabolites (e.g., glycogen, glucose, and lactate) in tissues before, during, and following a ∼1 d thermal challenge. Cellular and endocrine stress responses have been associated with summer field temperatures in salmonid fish (
Chadwick et al. 2015); thus, we also measured tissue heat shock protein 70 (HSP70) and predicted that these variables would increase with the thermal challenge. Given that the temperatures at which reductions in whole animal function occur are highly correlated with subcellular function (
Anttila et al. 2013), an understanding of the molecular-level effects of natural thermal cycles could provide insight into thermal tolerance. Our second objective was to determine if this thermal cycle, based on environmentally relevant temperatures, would affect subsequent acute thermal tolerance, as measured by the critical thermal maximum (CT
max), compared with fish maintained under control conditions. It was recently demonstrated that an appreciation of fishes’ true thermal sensitivity to climate warming requires an understanding of their upper thermal physiological limits (
Sandblom et al. 2016), making CT
max a particularly important dependent variable. We predicted that acute thermal tolerance would increase in Atlantic salmon exposed to a prior high temperature event.
Materials and methods
Animals
Atlantic salmon, post-smolt, were obtained from the Mactaquac Department of Fisheries and Oceans (DFO) Fish Culture Station (French Village, New Brunswick, Canada) in July 2012. These fish were the F1 progeny of early run, wild Saint John River fish, captured at the Mactaquac dam. As such, our fish were born and reared in the hatchery where they were acclimated to freshwater, held at 10 °C (±1 °C), 92% dissolved oxygen (DO), and a natural photoperiod. Fish (
n = 57) were transported to the Harold Crabtree Aqualab at Mount Allison University (New Brunswick, Canada), and divided among cylindro-conical holding tanks maintained at 12 °C (±1 °C), to obtain a stocking density of approximately 21 kg/m
3 (
Turnbull et al. 2005). Within 2 h of arrival, fish were treated with an acute salt bath as a preventative measure against fungus and external parasites that could result from the stress of transportation and handling. Salinity was quickly (i.e., over a few minutes) raised to 20 parts per thousand (ppt) in the holding tanks and maintained for 1 h. We then decreased salinity back to 10–12 ppt within 1 h and gradually returned fish to 0 ppt over 16 h. Atlantic salmon were maintained at a natural photoperiod with DO levels >85% and fed 3 and 5 mm commercial pellet feed (Corey Nutrition Company) ad libitum every other day. After approximately 2 weeks, when all fish had begun to eat, the temperature was increased 2 °C/d at a rate of 0.1 °C/h, from 12 to 15 °C. Fish were (mean ± standard error of the mean (SEM)) 596 g ± 15.3 g, 38.6 cm ± 0.34 cm long, with a condition factor (weight/length
3) of 0.0106 ± 0.0002 and held at 15 °C (±1 °C) for a minimum of 2 weeks before experiments began. Water temperature was monitored using an iBCod temperature logger (±1 °C, 15 min interval, Alpha Mach Inc.) and DO was measured daily (7.5–10 mg/L; YSI Pro 20; Xylem Inc.).
All experiments proceeded according to the Canadian Council on Animal Care guidelines approved by the Mount Allison University Animal Care Committee (MTA Protocol 12-09).
Temperature profiles
The thermal cycle (
Fig. 1) was derived from hourly temperature records obtained from the Miramichi River Environmental Assessment Committee (MREAC) in July 2010 so that we could approximate the thermal conditions (e.g., peak and rate) on a typical summer day in one of North America’s most productive Atlantic salmon rivers (
DFO 2013). We used hatchery fish in this study, recognizing that these fish may not be representative of the resident wild population. The thermal cycle began at 15 °C and increased to 26 °C at a rate of 0.82 °C/h; the temperature then decreased at a rate of 0.6 °C/h to return to 15 °C over 34 h. We exposed fish to these environmentally relevant temperatures and then assessed recovery. Fish were fasted 24 h before experiments began and during the thermal cycling.
Sampling
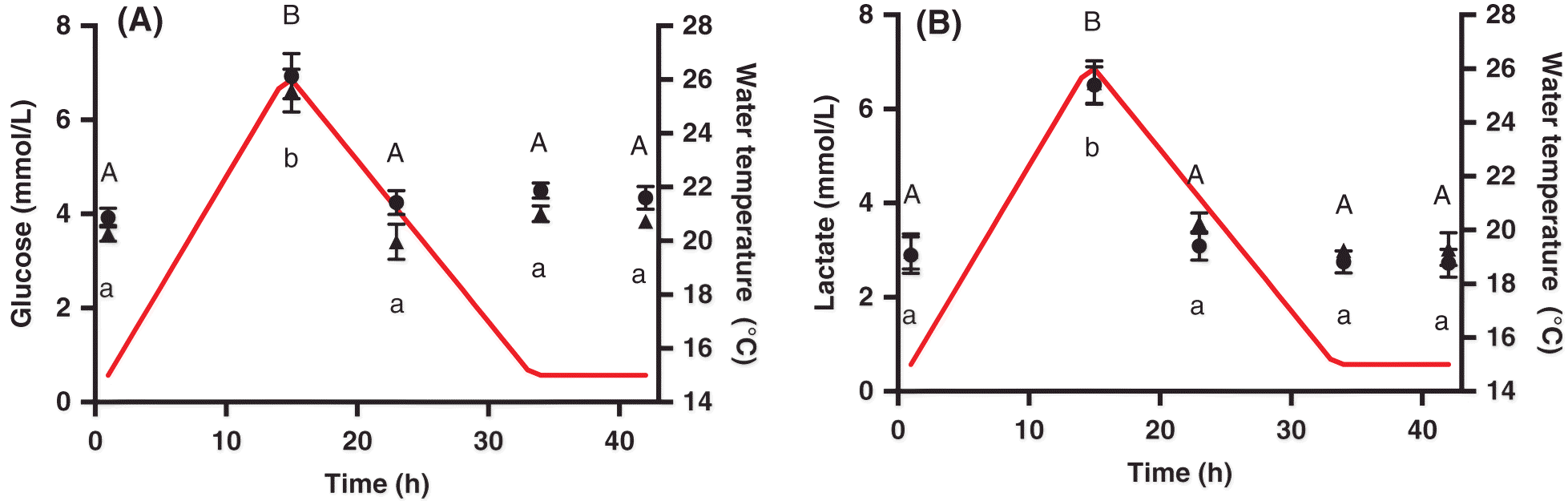
Four fish were transferred from the holding tanks to 300 L experimental tanks 24 h before experimentation with each tank representing a sampling point. This transfer was repeated once for n = 8 for each time point. Fish were terminally sampled per treatment: prior to heating (i.e., control, 0 h); at the temperature peak (15 h); 8 h following the thermal peak (23 h); when the temperature first returned to 15 °C (recovery, 34 h); or 8 h into recovery (42 h). Individual fish were anesthetized in an aerated, buffered solution of tricaine methanesulfonate (0.25 g/L; MS-222; Sigma-Aldrich, Oakville, Ontario, Canada), measured (±1 mm), and weighed (±0.1 g). Blood (5–10 mL) was sampled from the caudal vein with a 21 gauge needle, rinsed with heparinized Cortland’s saline (in mmol/L: 124.1 NaCl; 5.1 KCl; 1.9 MgSO4; 1.5 Na2HPO4; 11.9 NaHCO3; 1.1 CaCl2; 100 units/mL heparin), and immediately placed on ice. The spinal cord was then severed, and heart (ventricle), red muscle, white muscle, and liver samples were collected and immediately flash frozen in liquid nitrogen. Blood samples were analysed for whole blood glucose (One Touch Ultra 2 meter, LifeScan, Canada) and lactate (Arkray Lactate Pro meter, Fact Canada) concentrations and then centrifuged at 4 °C at 5000 rpm for 3 min to separate red blood cells (RBC) and plasma, which were immediately flash frozen in liquid nitrogen and stored at −80 °C.
HSP70 analysis
We used immunoblotting techniques to assess the relative concentrations of HSP70 in the heart, liver, red muscle, white muscle, and RBC samples. Soluble protein was extracted from the samples as performed by
LeBlanc et al. (2011) and assayed using the BioRad DC Protein Assay based on the Lowry method. HSP70 levels were determined in samples (15 μg soluble protein, within the dynamic range of our chemiluminescent detection agent) as performed by
Kolhatkar et al. (2014). Each gel contained the same Atlantic salmon RBC sample to express all other samples relative to this internal standard and to allow comparison across gels. Membranes were incubated in polyclonal rabbit affinity purified HSP70 primary antibody (AS05 061A; Agrisera, Vännäs, Sweden) at a concentration of 1:5000 in 1% milk powder tris-buffered saline with Tween20 (TBS-T) solution for 1 h at room temperature. This antibody is specific to the inducible isoform of salmonid HSP70 and does not detect the constitutive protein, providing a powerful tool to assess induction of HSP70 with thermal challenge. The secondary antibody was goat anti-rabbit immunoglobin G-horseradish peroxidase (IgG-HRP) (SAB 300, Enzo Life Sciences, New York, USA) used at a concentration of 1:5000 in 1% milk powder TBS-T solution for 1 h at room temperature. Chemiluminescent detection of protein bands was performed using ECL Select (GE Healthcare, Buckinghamshire, UK). Blots were imaged using a VersaDoc™ MP 4000 Molecular Imager (Bio-Rad), analysed using Image Lab
® software, and expressed relative to the internal standard facilitating qualitative comparisons of constitutive levels and fold-changes among tissues.
Metabolite extractions and assays
Frozen heart and red and white muscle tissues were ground under liquid nitrogen, followed by the addition of perchloric acid (8% PCA with 1 mmol/L EDTA). Samples were vortexed and centrifuged at 5 °C for 3 min at 10 000 rpm, and the supernatant was neutralized to pH 7 with NaOH and centrifuged as before for 1 min. The resulting supernatant was stored at −80 °C until processing. For plasma metabolite extraction, the 8% PCA solution did not contain EDTA. Liver glycogen was assayed as described by
Clow et al. (2004), and samples were read on a VERSAmax Tunable Microplate Reader (Molecular Devices Corporation) at 340 nm until absorbance stabilized. Hexokinase (25 μL) was then added to each well, and the absorbance was read after 15–25 min.
Plasma, and heart and white muscle lactate were analysed using an NADH-linked assay in a microplate at 340 nm with a VERSAmax tunable microplate reader, using a glycine buffer commercial assay kit (Sigma-Aldrich Canada Ltd.).
CTmax
We assessed the acute thermal tolerance of Atlantic salmon using an established CT
max protocol, recording the temperature when the fish lost equilibrium after a rapid heat ramp (
Becker and Genoway 1979;
Fangue et al. 2006;
Beitinger and Lutterschmidt 2011). Fish were lightly anaesthetized (0.083 g/L buffered MS-222), weighed (±1 g), measured (±1 mm), and then transferred to experimental boxes (40 cm × 13.5 cm × 11 cm) with a clear Plexiglas
® lid. They were left to recover in the experimental blackened PVC boxes for 24 h at 15 °C ± 1 °C before experiments began. Each box had an air stone with water flow at 4 L/min, and the DO remained >85% during all trials. CT
max trials for all control fish were performed at 0900, whereas trials for heat-shocked fish were performed at 0900 or 1300. We found no statistically significant differences between the CT
max values of heat-shocked fish that were done at the different times of day (
p = 0.5; two-tailed, unpaired
t test).
Atlantic salmon were exposed to either control conditions or a heat shock event as described previously. The control fish (15 °C) were left in the boxes for the same amount of time as the fish exposed to the heat shock. After exposure to the control condition or heat, the temperature was set to increase at a rate of 0.33 °C/min (
Becker and Genoway 1979) until the fish lost equilibrium for 2 consecutive seconds. The actual rate of heating in our experimental trials was 0.314 ± 0.006 °C/min. The temperature at which the fish lost equilibrium was recorded, and the temperature was immediately decreased over 20 min to 15 °C.
Statistical analyses
Statistical analyses were performed using SPSS Statistics software (version 19; IBM, Chicago, Illinois, USA). Before analysis, all data were checked for violations of assumptions using the Kolmogorov–Smirnov test for normality and the Levene’s test for homogeneity of variance. If data failed these assumptions, they were transformed using either a square root or logarithmic transformation. We used one-way ANOVAs to determine differences over time in our dependent variables. We used Tukey’s post hoc tests to determine differences among sampling points. We performed a logistical regression analysis (R Studio 3.3.2) to determine possible differences in percent mortality in the CTmax tests. For all analyses, the fiducial limit of significance was set to 0.05. All data are presented as mean ± SEM.
Discussion
Here, we describe the time course of cellular and metabolic responses in Atlantic salmon to a short-term, acute thermal event representative of field conditions. Our recent work in salmonids has shown distinct physiological and biochemical changes after several days of diel thermal cycling (
Callaghan et al. 2016;
Tunnah et al. 2017), and we were therefore interested in more detailed stress responses after ∼1 d of a field-relevant heat event. We also assessed whether this heat challenge would affect tolerance to a subsequent heat shock in terms of CT
max. We expected to observe signs of both cellular and physiological stress in these fish, and this prediction was supported. Contrary to our second prediction, we found no significant effect of a prior heat shock on CT
max.
A decrease in energy reserves may also be indicative of a stressed state. Glycogen is an energy storage molecule that can be broken down into glucose in times of high-energy needs, and thus we used this metabolite as an indication of energy status during the heat event. Despite the lack of any obvious decrease in liver glycogen over the heat shock, we did observe signs that the fish were mobilizing energy reserves, as both plasma and whole blood glucose increased. Similarly, white muscle lactate was elevated at the peak of the thermal cycle when metabolic demands should be elevated. Stressed salmon have increased energy demands (
Vijayan et al. 1990) and cease feeding at high temperatures (
Breau et al. 2011); thus, the temperature increase experienced here would necessitate the mobilization of some energy stores over the 1 d heat shock. Accordingly, fish with a higher condition factor and more energy reserves may be more thermally tolerant, as we observed here with the positive correlation of CT
max with condition factor. Recently,
Callaghan et al. (2016) showed that 1 d of sub-critical heat exposure in rainbow trout (
Oncorhynchus mykiss) depleted glycogen stores; however, after multiple days of thermal cycling, fish adopted an anabolic phenotype and replenished energy reserves. Such metabolic plasticity would equip Atlantic salmon to cope with subsequent heat exposures and would likely be even more important as temperatures approached their upper limits.
HSP70 induction in brook trout has been correlated with population-specific thermal tolerances (
Stitt et al. 2014) and thermal limits (
Chadwick et al. 2015), leading to the suggestion that HSPs are useful markers to determine ecological thresholds in salmonids (
Chadwick et al. 2015). Notably, two highly stress-inducible HSPs, HSP70 and HSP30, significantly increased in wild Miramichi Atlantic salmon during heat events when water temperatures reached 23 °C (
Lund et al. 2002). This cellular stress response coincides with the upper threshold for feeding in Atlantic salmon (
Breau et al. 2011) and is likely indicative of protein damage (
Tunnah et al. 2017).
Fader et al. (1994) showed that HSPs were induced in several species of fishes with natural seasonal variations in temperature and there have been subsequent studies in a variety of species on this theme (e.g.,
Podrabsky and Somero 2004;
Todgham et al. 2006;
Narum et al. 2013). Tissue-specific HSP70 induction patterns were observed in Atlantic salmon experiencing natural diel thermal cycles that were distinct from an acute heat shock (
Tunnah et al. 2017). Here, we measured induction profiles of HSP70 throughout the heat event, rather than just before and after, in five tissues to understand immediate responses to an ecologically relevant heat stress. The induction was similar in magnitude in all tissues, but we did observe distinct time courses of the heat shock response in each tissue that may reflect tissue-specific sensitivities to thermal stress. For example, the early induction of HSP70 in the heart and red muscle is suggestive of early damage in these highly aerobic tissues.
In this study, HSP70 remained elevated into recovery in all tissues. In brook trout,
hsp genes remained elevated at least 48 h following an acute heat shock (
Lund et al. 2003), suggesting that recovery from the heat shock response does not occur before the next day of heat cycling. On the one hand, maintaining a high titer of inducible HSPs in tissues will offer protection against thermal denaturation and may precondition tissues against a second thermal insult. Indeed, the induction of HSPs has been correlated with thermal preconditioning in fishes (
Feminella and Matthews 1984;
Hightower et al. 1999), whereby exposure to a prior heat event protects the animal from a subsequent higher temperature that may otherwise be lethal. However, our CT
max data do not support this possibility, in that fish exposed to ∼1 d thermal cycle were not more thermally tolerant than fish maintained under control conditions. In contrast, given the energetic cost of protein synthesis in cells (∼11–42% of total energy expenditure;
Carter and Houlihan 2001), a robust heat shock response represents a significant cost over multiple days/months of warming. Although we found no evidence of enhanced thermal tolerance with 1 d of thermal cycling, several days of warm, ecologically relevant diel cycling has recently been shown to increase CT
max in wild Atlantic salmon (
Corey et al. 2017). We note that our mortality rates were high following CT
max trials and this may be linked to the depletion of cardiac glycogen stores after a single thermal cycle, as we observed previously (
Callaghan et al. 2016), and (or) irreversible cell and tissue damage. These factors could increase the sensitivity of the heart to arrhythmias at the CT
max temperature (
Anttila et al. 2013). Although Atlantic salmon demonstrated cardiac plasticity with temperature (
Anttila et al. 2014), their capacity for physiological plasticity in their upper thermal limits may be small relative to their lower thermal limits, as observed in other fish (
Sandblom et al. 2016).
In July 2010, the water temperature of the Miramichi River remained above 23 °C, the critical temperature of Atlantic salmon, for 18 consecutive days (
DFO 2012). High mortality and the eventual closing of the recreational fishery followed this temperature event. Here, we show that a single thermal event, representative of summer water temperatures in the Miramichi River, resulted in heat shock and physiological stress responses, and increased energy demands with no effect on acute thermal tolerance. Atlantic salmon can cope with this short-term heat shock, but this challenge may compromise the ability to cope with a later acute heat stress. This conclusion is supported by the lack of any obvious thermal preconditioning in wild Atlantic salmon experiencing several days of thermal cycling and then subjected to an acute heat shock (
Tunnah et al. 2017). Recognizing the thermal limits, tolerance, and physiological consequences of thermal stresses faced by Atlantic salmon is critical to manage this important species and to understand the biological impacts of recorded and anticipated increases in temperature.