Bat handlers, bat bites, and rabies: vaccination and serological testing of humans at risk
Abstract
Introduction
| Recognized species | Common name | Phylogroup* | Distribution |
|---|---|---|---|
| Lyssavirus aravan | Aravan virus (ARAV) | I | Eurasia |
| Lyssavirus australis | Australian bat lyssavirus (ABLV) | I | Australia |
| Lyssavirus bokeloh | Bokeloh bat lyssavirus (BBLV) | I | Europe |
| Lyssavirus duvenhage | Duvenhage virus (DUVV) | I | Africa |
| Lyssavirus hamburg | European bat lyssavirus 1 (EBLV-1) | I | Europe |
| Lyssavirus helsinki | European bat lyssavirus 2 (EBLV-2) | I | Europe |
| Lyssavirus gannoruwa | Gannoruwa bat lyssavirus (GBLV) | I | Sri Lanka |
| Lyssavirus ikoma | Ikoma virus (IKOV) | III | Africa |
| Lyssavirus irkut | Irkut Virus (IKRV) | I | Eurasia |
| Lyssavirus khujand | Khujand virus (KHUV) | I | Eurasia |
| Lyssavirus lagos | Lagos bat virus (LBV) | II | Africa |
| Lyssavirus lleida | Lleida bat virus (LLEBV) | III | Western Europe |
| Lyssavirus mokola | Mokola virus (MOKV) | II | Africa |
| Lyssavirus rabies | Rabies virus (RABV) | I | Global† |
| Lyssavirus shimoni | Shimoni bat virus (SHIBV) | II | Africa |
| Lyssavirus formosa | Taiwan bat lyssavirus (TWBLV) | I | Asia |
| Lyssavirus caucasicus | West Caucasian bat virus (WCBV) | III | Eurasia |
Note: Distributions are approximate.

Vaccines and titres
Working with bats and managing exposures
Prophylaxis for unvaccinated persons
Prophylaxis for persons previously vaccinated for rabies
In the context of rabies, are certain bats more “dangerous” than others?
Rapid immunochromatographic test kits for rabies
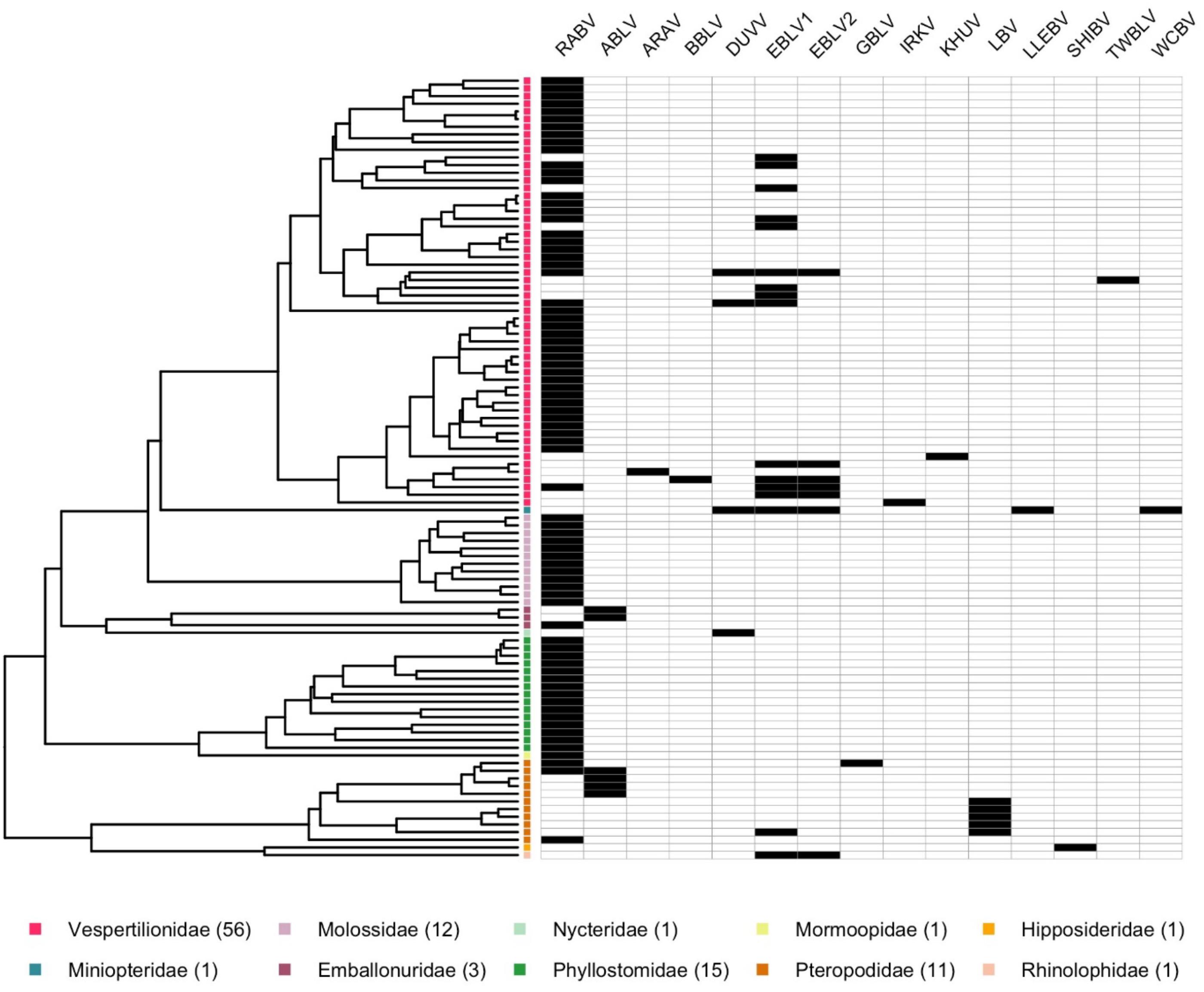
Lyssaviruses
Nine critical take home messages about rabies for bat biologists
Acknowledgements
References
Appendix A. Always seek medical advice after a bat bite
Appendix B. Carry proof of PrEP and latest titre
Appendix C. Do not refuse PEP after contact with a known rabid animal. Seek post-PEP serological testing to ensure you are immunocompetent
Appendix D.
Information & Authors
Information
Published In

History
Copyright
Data Availability Statement
Key Words
Sections
Subjects
Plain Language Summary
Authors
Author Contributions
Competing Interests
Funding Information
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
There are no citations for this item
