Introduction
The Atlantic lobster (
Homarus americanus), fished across 40 managed lobster fishing areas (LFAs) in the Atlantic provinces and Québec, is one of the most important iconic seafood exports of Canada and is worth more than $1 billion (CAD) annually (
Department of Fisheries and Oceans (DFO) 2014) with sustained increases in market demands.
Since the early 2000s, there has been a significant increase (from historically 5–10% to over 30–40% in recent years) in the proportion of soft-shelled lobsters landed in southwest Nova Scotia (SWNS) (
ASHS 2014). Soft-shelled lobsters have lower meat yields (
Rosa and Nunes 2004) and poorer survivability during handling and transportation (
Factor 1995), which have negative economic consequences to the lobster industry, as well as affecting consumer confidence (
Jaffry et al. 2004;
Bremner 2007).
Lobster shell hardness is directly related to the timing of their molting stages, a natural process whereby they shed their exoskeleton, allowing them to grow (
Cobb and Phillips 2012;
Howell 2012). While they molt, a new, soft shell is formed, and lobsters actively take in water to expand and stretch this soft exoskeleton (
Glenn and Pugh 2006;
Laufer et al. 2013). Therefore, recently molted lobsters have reduced meat yield and excess water content, which is sometimes undesirable to consumers (
Wang and Mcgaw 2014). In the days to weeks following molting, excess water is replaced with new tissue growth and re-mineralization of the newly formed exoskeleton, producing harder shells (
Howell 2012).
Environmental and ecosystem-related factors such as water temperature and diet can affect the molt timing and influence shell hardness and product quality (
Waddy and Aiken 1992;
Mikami 2005;
Hammond et al. 2006). Lobster molting occurs most frequently in the summer months, with evidence to suggest that there are some regional differences. For example, Comeau and Savo (
Kunkel et al. 2012) reported that most lobsters in their study molted between early July and early September in the southern Gulf of St. Lawrence, while Campbell (
Aiken and Waddy 1986) suggested that the majority of lobsters molted during the period from August to October in the Bay of Fundy.
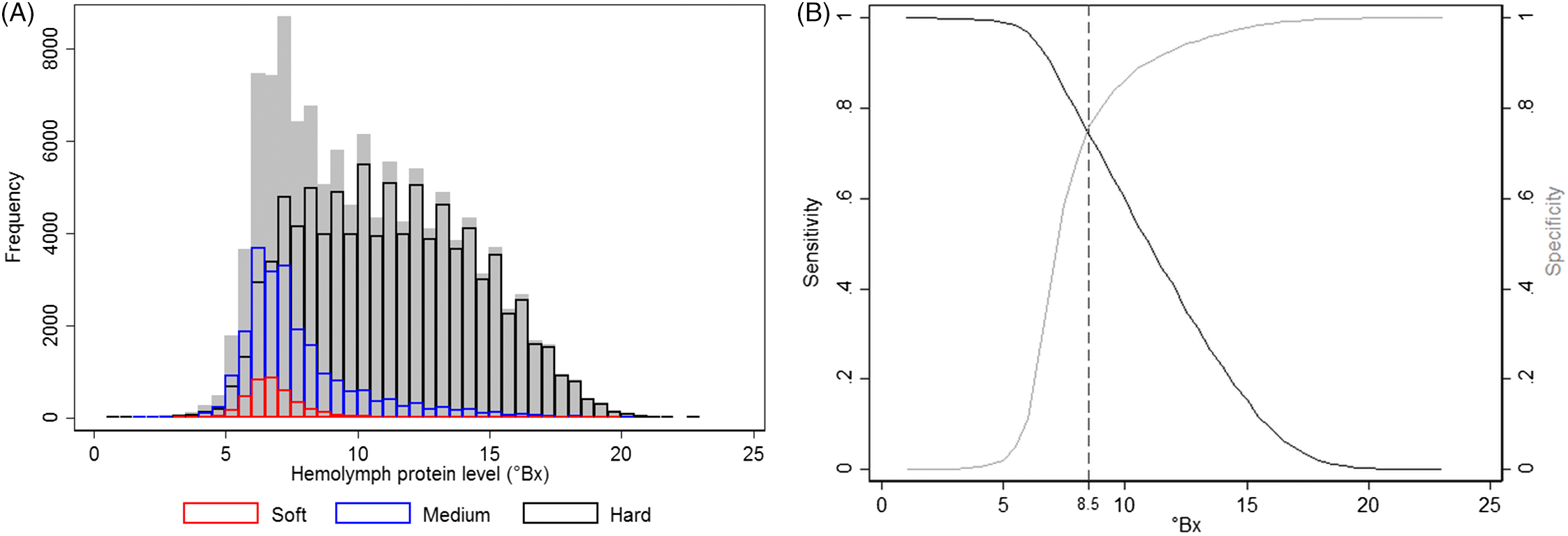
Physiologically, an important lobster-related indicator for the molting stage and shell hardness is their hemolymph total protein level (
Cobb, 1976). A rapid decrease in hemolymph protein concentrations occurs after molting when the lobster absorbs a significant amount of water to expand the capacity of its newly formed soft shell, which dilutes the hemolymph protein values. The concentrations of hemolymph protein, measured via refractometry, increase thereafter, peaking just prior to the next molt, when muscle tissue reaches its maximum capacity within the existing shell (
Barlow and Ridgway 1969;
Mercaldo-Allen 1991;
Wang and Mcgaw 2014).
Since 2004, the Atlantic Lobster Molt and Quality (ALMQ) monitoring project (
www.lobstermoult.ca) has been routinely collecting information on lobster shell quality, including lobster-related characteristics and hemolymph protein concentrations. The primary objective of the present study was to use the information collected by the ALMQ monitoring project to investigate the increased prevalence of soft-shelled lobsters in eastern Canada. More specifically, the present study attempted to describe spatio-temporal patterns of soft-shelled lobsters in SWNS, and identify lobster-related (e.g., sex and size) and environmental (sea surface temperatures (SST)) factors associated with shell quality.
Discussion
The present study sought to provide preliminary insights into the spatio-temporal patterns of soft-shelled lobster prevalence in recent years on the east coast of Canada, and attempted to identify environmental and lobster-related factors that could explain such trends. The ALMQ database, one of the largest datasets of lobster catches in the world, provided useful data from 2004–2014 for the present study. The prevalence of soft-shelled lobsters varied annually (ranging from 9 to 38%) and among the LFAs. The analytical part of the present study focused on LFAs 33 and 34, as the majority of data originated from these LFAs and, combined they represent approximately 40% of the annual Canadian lobster production.
Hemolymph protein levels are not an entirely adequate predictor of shell hardness but can be useful as an indicator of shell quality, as in over 80% of cases they accurately identified shell hardness. However, caution should be exercised when interpreting these results, as several other factors may affect serum protein concentration, such as lobsters’ diets (
McLeese 1972;
Hagerman 1983) and live wet weight (
Stewart et al. 1967). Taking these into consideration, one potential explanation for the inconsistency in predicting shell hardness based only on °Bx is that changes in protein levels during the molt cycles are more important for shell hardness than are their absolute values at any given point. As only a single value was recorded for each lobster, we were not able to assess individual changes in protein levels over time. A future longitudinal study with repeated measures of °Bx may help explain the relationship between hemolymph and shell hardness, and the role it may have on survivability of lobsters during storage and transportation.
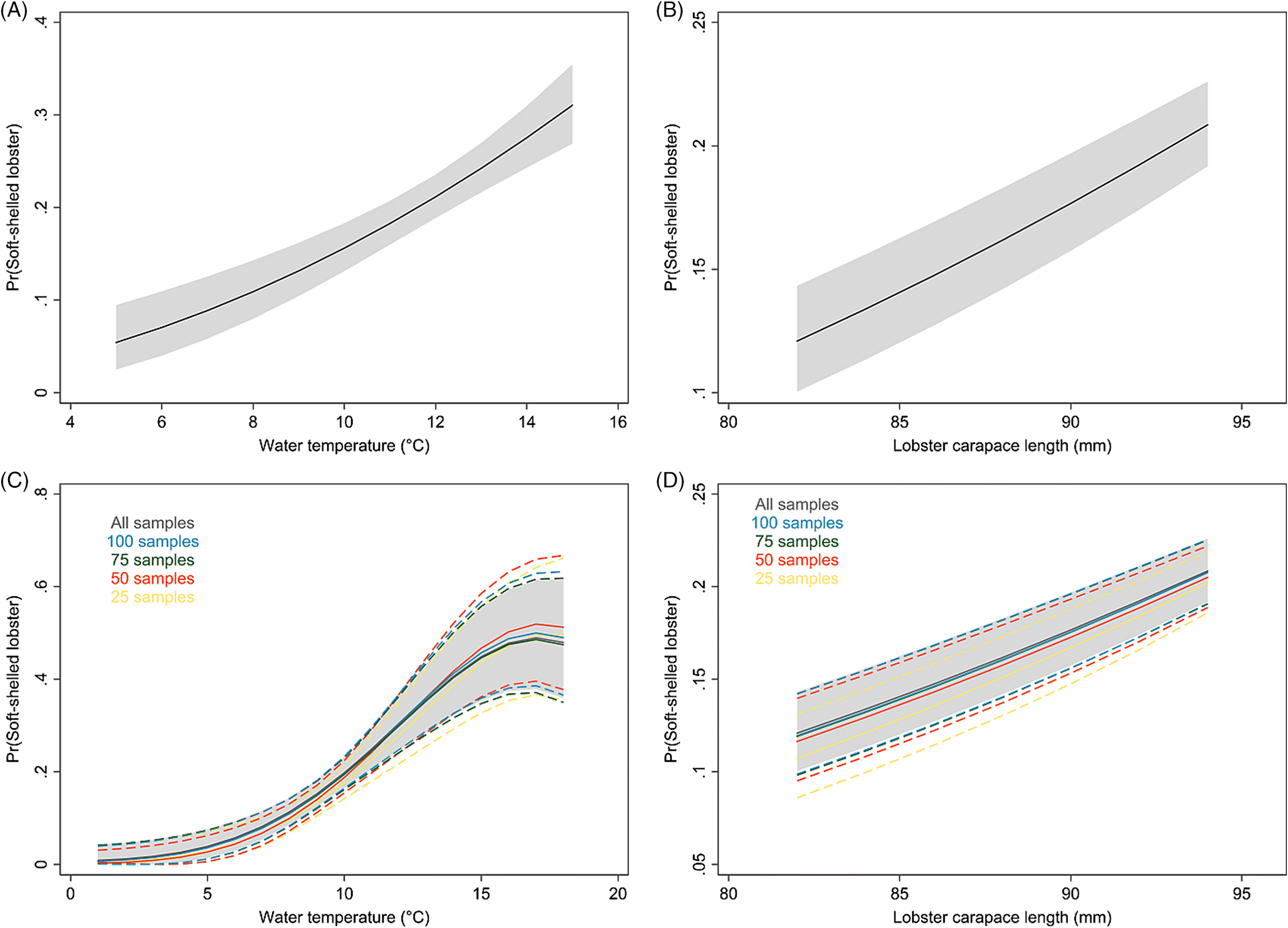
The present study documents an increase in the prevalence of soft-shelled lobsters in SWNS. Our findings also suggest that larger lobsters are more likely to have soft shells, and that males are more likely to have soft shells than females. We do not have full biological explanations for these associations; however, it is known that sexually mature female lobsters molt less frequently than males (
Cobb and Phillips 2012). Common knowledge dictates that larger lobsters do not molt as often as smaller lobsters, and therefore we would expect to have an overall higher prevalence of soft shells in small animals. That may be true when comparing very young with mature lobsters, but the present study included mostly market-sized lobsters (i.e., adults). In addition, the frequency and duration of molting also influence shell hardness, which we could not evaluate in the present study, as additional data for individual lobsters were not available. Lobster carapace length for the samples in the database varied between fishing and non-fishing seasons, due to a preference for the legal cut-off (82.5 mm) for landed lobsters’ size; during the fishing season, approximately 5% of sampled lobsters were of sub-legal size. This should be considered when interpreting the effect of size on shell hardness, as it may have created an upward bias in the estimate. The study also indicated a high level of variability among fishing locations and years on the prevalence of soft-shelled lobsters. However, inter-year variability was more pronounced, suggesting differences in climatic conditions across the years. Lagged SST was an important predictor for shell quality, and surprisingly, inter-year variability was not fully accounted for, even when water temperature was included as a predictor, suggesting a role for some unmeasured factor(s) on the differences in soft-shelled lobsters over the study years. Molting probability is highly temperature dependent, with increased water temperature being associated with a higher rate of molting (
Templeman 1936;
McLeese 1956;
Schmalenbach and Buchholz 2013), and thus a higher probability that a captured lobster will have a soft shell. We used SST in the present study as a surrogate for water temperature, as studies comparing remote-sensed SSTs with in-situ records have suggested a high level of agreement between the two (
Castillo and Lima 2010;
Williams et al. 2013).
The average water-temperature 4–6 weeks prior to sampling was the best predictor for shell quality among the temperature metrics evaluated. In the results from the model with short-term temperature effect, we observed strong seasonal trends in the prevalence of soft-shelled lobsters that generally coincided with the fishing season for LFAs 33 and 34. Based on temperature profiles, the best time for lobster fishing would appear to be from January to early February. However, the data suggest that the prevalence of soft-shelled lobsters is low from the beginning of the year through May or June.
Our results suggest that lower temperatures are associated with fewer soft-shelled lobsters. The initial motivation to include SST in our analysis was to investigate changes in fishing season periods or to assess the potential for dynamic fishing “windows” based on previous years’ SST and its lagged effect. Because the inter- and intra-year variability of the prevalence of soft-shelled lobsters were high and SST could not explain much of this variability, changes or dynamic fishing seasons are not recommended. Nonetheless, there might be potential to change the start date of the fishing season dynamically, year to year, depending on the average water temperature profiles 4–6 weeks prior to the start date. However, increased sampling efforts close to the start of the fishing season and data on total landings are needed to improve the confidence of our current estimates and to evaluate potential confounding due to variations in landed catch. Additionally, we do not know if landed soft-shelled lobsters were proportionally representative of the population from which they were sampled, which was an assumption we had to make when interpreting our results. If, however, a selection bias did exist where soft-shelled lobsters were preferentially caught (i.e., higher observed proportions of soft-shelled lobsters than were present in the population), we would expect prevalence to be influenced by the intensity of the landed catch in an area and would likely have observed a higher prevalence of soft-shelled lobsters outside of the fishing season than were actually present in the population.
Similarly, the AR1 coefficient (ρ) of 0.628 indicated a relatively strong correlation in the soft-shelled lobster prevalence from week to week, which decreased exponentially as the time periods increased, with a negligible correlation among lobsters caught six weeks apart. These findings indicate that if start of the fishing season was moved to February and continued until June, then we might expect a reduction in soft-shelled lobsters. In contrast, moving the fishing season by a fixed number of weeks in December, without considering recent water temperature profile, would likely have little effect.
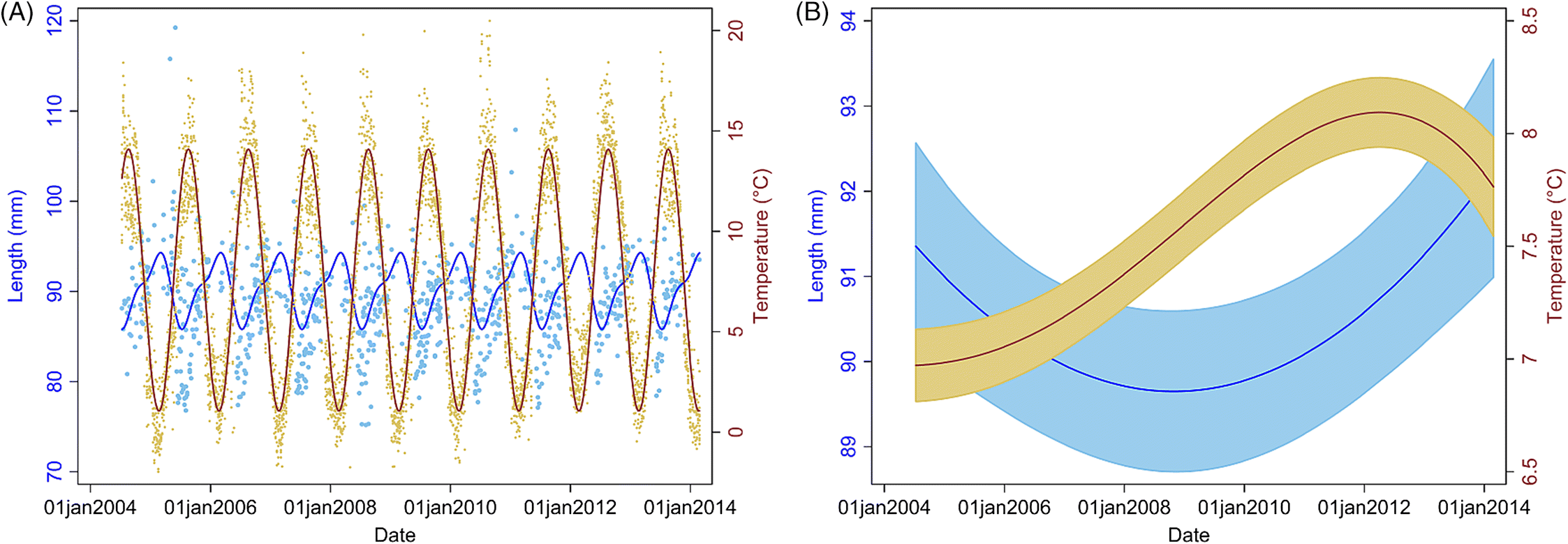
However, given the trend toward increasing temperatures long term and that our study shows an association of soft-shelled lobsters with an increase in SST, our model predictions indicate that this problem will likely persist and worsen in SWNS. Our findings also indicate that lobsters are getting larger over time (since 2006, size has been increasing), and anecdotal reports and published studies suggest that the abundance of lobsters is increasing in LFAs 33 and 34 (
Howell 2012;
Tremblay et al. 2013). These findings raise intriguing questions regarding the interplay among rising temperature, increasing lobster abundance and size, and increased prevalence of soft-shelled lobsters. Other unmeasured factors such as water depth, bottom temperature, and effect of spatial location for the samples are likely present and would help answer these questions. Future sampling strategies should consider sampling consistently in other LFAs and fishing locations, and sampling more frequently, both near the start of and during the initial weeks of the fishing season.
Atlantic lobsters in the coastal waters of southern New England, south of the LFAs in the present study, are under severe stress due to increasing water temperatures. This thermal stress has led to the development of an unusual syndrome in lobsters, known as epizootic shell disease (ESD), characterized by rapid degradation of the shell/cuticle. Stress from temperature, commercial exploitation, and ESD has resulted in massive declines in lobster stocks in southern New England waters (
Glenn and Pugh 2006;
Howell 2012;
Laufer et al. 2013). The direct commercial effect of ESD is that infected lobsters do not command a premier price due to necrosis of the carapace and claws. However, a more important biological effect could be the potential decline in egg production, as ESD is more prevalent in ovigerous females. Female lobsters do not normally molt while bearing eggs (
Shields and Sainte-Marie 2013), which could lead to death if lesions from ESD are severe, or they may molt prematurely, causing death of the larvae (
Kunkel et al. 2012). In contrast to these declines in lobster stocks in southern New England waters, studies have suggested an increase in lobster stocks in northern US waters and in LFAs on the east coast of Canada (
Howell 2012;
Tremblay et al. 2013).
Water temperature is the most important environmental variable, as it affects the growth, reproduction, metabolic rate, and survival of lobsters (
Cobb 1976;
Aiken and Waddy 1986;
Cobb and Phillips 2012). Studies indicate that ocean water temperatures are increasing globally (
Harvell et al. 2002;
Domingues et al. 2008;
Maynard et al. 2015,
2016), and our study reflects this trend with evidence of increasing water temperatures in SWNS in recent years. Increasing water temperatures in SWNS and the Gulf of Maine may provide a favorable environment for ESD to expand from southern coastal waters into these areas (
Maynard et al. 2016). Lobsters in these areas (LFAs 33 and 34) and the Bay of Fundy will likely be among the first to be affected in Canada. With ESD present in New England lobsters and the potential for emergence in lobsters in Canadian waters, the ongoing ALMQ project should be able to detect emergence of the disease and provide surveillance data for future studies.
More specific location data would have been required to add a range of environmental predictors to the study. Had detailed sampling locations for each observation been available, buffer zones could have been generated around each location to extract more precise environmental predictors (water depth and SST). Without individual sample locations in the database we were limited to using entire fishing locations to extract median values for our environmental predictors, greatly reducing the amount of information and variability in these predictors. The addition of higher resolution bathymetry (water depth) data would have enabled us to generate additional predictors, such as ocean floor slope, distance to the edge of the coastal shelf, and depth variability within buffer zones (surrounding sampled location points)—all potentially useful predictors. Similarly, using algorithms to predict bottom temperatures from the SST could add precision to the estimates (
Maynard et al. 2016). It should also be noted that for the temporal models with water temperature, we aggregated observations at the fishing location levels, and thus the estimates from these models should not be interpreted as individual-level estimates.
For spatio-temporal modeling, continuous sampling over space and time is very important. The ALMQ database contains large amounts of data (>130 000 entries); however, the temporal gaps in some areas and the lack of sampling in others forced us to restrict the analyses to a small portion of the lobster industry. LFAs 33 and 34 make up approximately 40% of the annual lobster landings in Canada, while 93% of the database samples come from these two LFAs. To better understand spatio-temporal trends across the whole fishery, more frequent sampling across all fishing locations would be required. Naturally, sampling more locations more frequently is associated with higher costs and increased use of resources. However, a tradeoff can be achieved by decreasing the number of samples per sampling event. Currently, the number of lobsters sampled per event ranges from 2 to 398, with an interquartile range from 125 to 199 (mean = 151, median = 125). This number could effectively be halved (with an aim to catch approximately 50–75 lobsters/sampling event) with little effect on model estimates.
Studies have also suggested an association between water temperature and catch size (
Fogarty 1988;
Drinkwater et al. 1996). Forecast models have been developed using water temperature as a predictor to suggest the best time to start fishing in the state of Maine, USA (
http://www.gmri.org/our-work/research/projects/gulf-maine-lobster-forecasting). Our findings suggest that similar forecast models for Atlantic Canada, with water temperature as a key predictor, may be helpful in forecasting the best time for fishing both in terms of catch size as well as to help lower the proportion of soft-shelled lobsters caught.