Cranial anatomy
Ankylosaurus shares multiple cranial features in common with its close relatives
Anodontosaurus,
Dyoplosaurus,
Euoplocephalus,
Scolosaurus,
Ziapelta, and
Zuul (
Vickaryous and Russell 2003;
Arbour and Currie 2013a;
Arbour et al. 2014a;
Arbour and Evans 2017) (
Figs. 2–
4,
Tables 1,
2,
Supplementary Material 1). All of these taxa have (where preserved) cranial sculpturing characterized by rectangular to hexagonal frontonasal caputegulae, a large hexagonal median nasal caputegulum (except
Ziapelta), a single loreal caputegulum, a single lacrimal caputegulum, and pyramidal squamosal and quadratojugal horns (
Fig. 3). They are all similar in palatal view as well, with a broad premaxillary beak, deep but thin vomers, curved tooth rows, anterolaterally oriented pterygoid flanges, a short and robust braincase, and laterally oriented paroccipital processes (
Fig. 2B).
Ankylosaurus is easily distinguished from other derived Laramidian ankylosaurines (the ankylosaurins,
Arbour and Currie 2016) based on aspects of its cranial ornamentation (
Figs. 3,
4). Unlike
Anodontosaurus,
Euoplocephalus, and
Scolosaurus, the keel on the anterior and posterior supraorbital osteoderms is continuous with the keel of the squamosal horn (
Fig. 4). The squamosal horn is proportionately longer in
Ankylosaurus compared with the squamosal horn of
Euoplocephalus or
Anodontosaurus, and is not posteroventrally curved as in
Scolosaurus or lateroventrally curved as in
Ziapelta (
Fig. 3). It lacks the furrows present on the squamosal horn of
Zuul. The median nasal caputegulum is proportionately large, similar to that in
Euoplocephalus. In
Ziapelta, the median nasal caputegulum is triangular with a posteriorly directed apex, and in
Ankylosaurus the median nasal caputegulum is hexagonal, as in
Anodontosaurus,
Euoplocephalus, and
Zuul. The loreal caputegulum in
Ankylosaurus is proportionately larger than in other Laramidian ankylosaurins, expanding onto the anterior surface of the premaxillary beak and overhanging the lateral surface of the maxilla (
Fig. 4).
Carpenter (2004) provided a modern diagnosis for
Ankylosaurus, noting multiple unique features of this taxon such as the lateral and posterior displacement of the external nares, and the greater number of maxillary and dentary teeth compared with other ankylosaurines. The greater number of teeth in
Ankylosaurus does not appear to be simply a reflection of larger body size in
Ankylosaurus; rather, a biplot of maximum (unworn) crown height vs. basal skull length in ankylosaurs illustrates that the teeth of
Ankylosaurus are proportionally quite small (
Table 1,
Figs. 2G,
5), and that the jaws of the animal could therefore accommodate more of them. Tooth size appears to be highly variable in
Ankylosaurus; the teeth of the largest skull (CMN 8880) are absolutely smaller than those of the smallest skull (AMNH 5214). The same variability is not seen in
Pinacosaurus grangeri, the two representative specimens of which plot equidistant above the regression line (
Fig. 5).
Some of the diagnostic characters
Carpenter (2004) identified are also present in closely related ankylosaurins, which we comment on here. A posterodorsolaterally directed squamosal horn, posteroventrolaterally directed quadratojugal horn, and cranial ornamentation divided into polygons are present in all Laramidian ankylosaurins, not just
Ankylosaurus (
Fig. 3). In
Ankylosaurus, the maximum width of the maxillary tooth rows at their posteriormost extent is similar to the maximum width of the premaxillary beak (
Fig. 2B). This feature is also present in
Anodontosaurus (e.g., TMP 1997.132.1),
Ziapelta (
Arbour et al. 2014a), and
Zuul (
Arbour and Evans 2017). Finally, the quadrate process of the pterygoid is directed posterolaterally in
Ankylosaurus, and this feature is also present in
Anodontosaurus, Euoplocephalus, and
Zuul (
Arbour and Currie 2013a;
Arbour and Evans 2017).
The narial region in
Ankylosaurus warrants extra attention, as it has undergone an extreme transformation relative to other Laramidian ankylosaurins. Ankylosaurines have unusual nasal anatomy, with complex nasal vestibules, convoluted looping nasal passages, and a high degree of vascularisation in the posterior portions of the airway (
Hill et al. 2003;
Witmer and Ridgely 2008;
Miyashita et al. 2011). The border of the external naris in ankylosaurines is typically demarcated by a distinct edge on the premaxilla and the external surface of the supranarial caputegulum. Posterior to the external naris is the nasal vestibule, a concave region roofed by the nasals. The nasal vestibule contains one or more openings, the narial apertures, for the airway and sinuses (
Hill et al. 2003). Some Mongolian taxa, such as
Saichania and
Pinacosaurus, possess three or more narial apertures (
Hill et al. 2003;
Arbour and Currie 2016);
Anodontosaurus,
Euoplocephalus,
Zuul, and
Ankylosaurus have a single folded narial aperture.
The external nares of most ankylosaurines are anteriorly to anterolaterally oriented (e.g.,
Maryańska 1977;
Vickaryous et al. 2004;
Arbour and Currie 2016), but the external nares in
Ankylosaurus are located posteriorly relative to other ankylosaurs, and open ventrolaterally (
Figs. 2,
4).
Coombs (1971) suggested that this shift in external naris position resulted from the expansion of the nasals,
Maryańska (1977) suggested it was overgrowth of cranial ornamentation, and
Carpenter (2004) suggested it represented expansion of the internal sinuses. Unlike most other ankylosaurins, the external naris in
Ankylosaurus is roofed by the loreal caputegulum, which takes on a bulbous, inflated appearance compared with that in
Euoplocephalus,
Anodontosaurus,
Ziapelta, and
Zuul (
Figs. 2,
4). The supranarial caputegulae, which form an arched, rugose border above the external nares in most ankylosaurins, do not contribute to the border of the external nares at all in
Ankylosaurus. Instead, they are reduced in size, and are flush with the skull rather than protruding anteriorly (
Fig. 4). The loreal caputegulum covers part of the nasals in most ankylosaurins, and the supranarial caputegulae cover part of the premaxilla. As such, the interpretations presented by
Coombs (1971) and
Maryańska (1977) are both correct: the morphology of the caputegulae in this region suggests that the nasal bones, represented by the loreal caputegulae, have expanded anteriorly and pushed the supranarial caputegulae medially and anteriorly until they occupy only the tip of the snout.
One other ankylosaurine lacks anteriorly oriented external nares. Like
Ankylosaurus, the external naris of
Nodocephalosaurus is roofed by the loreal caputegulum, but this is ridge-like and laterally protruding, and the supranarial caputegulum is a prominent, anteriorly directed ridge (
Sullivan 1999;
Arbour and Currie 2016;
Fig. 4). The boundaries of the external naris of
Nodocephalosaurus are difficult to determine because of breakage in the only known specimen, but the naris likely faced only laterally, and not ventrolaterally.
Nodocephalosaurus has not been recovered as a close relative of
Ankylosaurus (e.g.,
Thompson et al. 2012;
Arbour and Currie 2016), and so a posterior placement of the external nares was most likely independently evolved in these taxa.
The unusual placement of the external nares in
Ankylosaurus is partially the result of anterior expansion of the loreal caputegulae (and therefore the nasals) and reduction of the premaxillae and the premaxillary contribution to the external naris. However, there also appears to have been a posterior shift in the external naris, and not just a lengthening of the snout anterior to the naris. The external naris in
Ankylosaurus is bordered posteriorly by the lacrimal caputegulum, which covers the lacrimal and part of the maxilla in other ankylosaurins (
Arbour and Currie 2013a). This caputegulum does not abut the external naris in
Anodontosaurus,
Euoplocephalus,
Ziapelta, or
Zuul. Additionally, in ventral view, the anterior edge of the tooth row (located entirely on the maxilla) is located at the midpoint of the external naris in
Ankylosaurus but is aligned with the posterior corner of the supranarial caputegulae (and therefore external naris) in
Euoplocephalus.
This expansion of the nasals and loreal caputegulae, and reduction of the dorsal surface of the premaxillae, may also have influenced the morphology of the premaxillary palate.
Coombs (1971) noted that
Ankylosaurus had a narrower beak than
Euoplocephalus, which may in part reflect the widening of the nasals and loreal caputegulae as well as a narrowing of the premaxillary beak itself (
Ősi et al. 2017). The expansion of the nasals and posterior shift of the external naris must also have modified the course of the internal nasal passages. AMNH 5895 is broken in such a way that the internal nasal passages are partially revealed in cross section.
Witmer and Ridgely (2008) showed that the structures in
Euoplocephalus previously identified by
Coombs (1978a) as sinuses were instead a continuous looping airway; the channels visible in AMNH 5895 most likely also represent a continuous looping airway, although it is difficult to interpret the course of this airway at present in the absence of computed tomography data for this taxon.
All three of the known skulls of
Ankylosaurus are very similar where the preserved areas overlap (
Carpenter 2004;
Fig. 2), and there is apparently slightly less intraspecific variation than that observed for
Euoplocephalus or
Anodontosaurus (
Arbour and Currie 2013a), although the small sample size undoubtedly influences this. The only notable differences between AMNH 5214 and CMN 8880 are the overall size, the sharpness of the squamosal horn apex, and the distinctiveness of the nuchal caputegulae (
Fig. 3). CMN 8880 has blunter squamosal horns than AMNH 5214, and the boundaries of the nuchal caputegulae are less distinct. Larger individuals of
Euoplocephalus have more rounded squamosal horns than smaller individuals (
Arbour and Currie 2013a), so this may represent ontogenetic variation in both
Euoplocephalus and
Ankylosaurus.
Postcranial anatomy
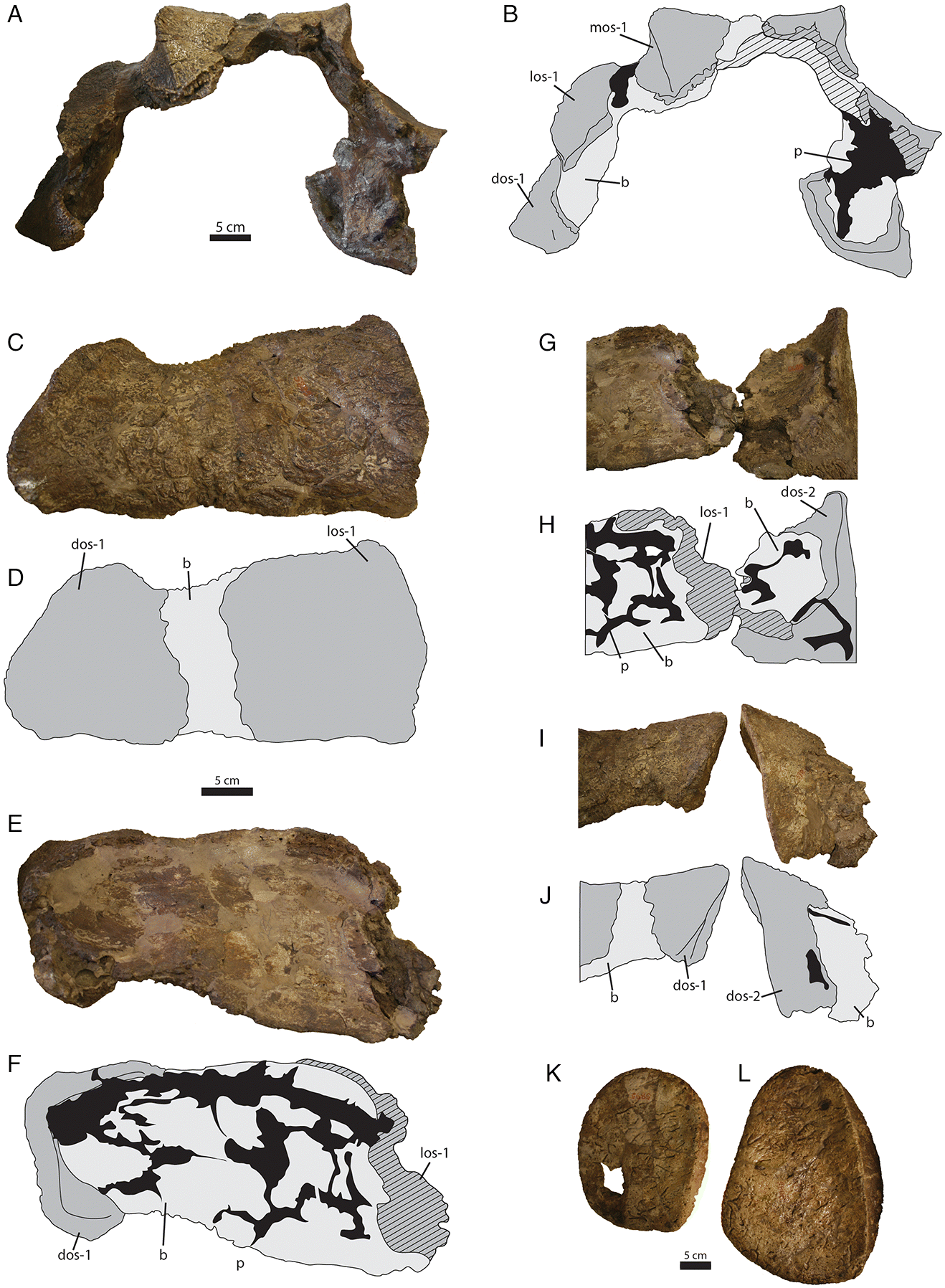
A unique trait of
Ankylosaurus described by
Carpenter (2004) concerns the morphology of the cervical half rings. Ankylosaurid half rings are unusual yoke-like structures that overlie the cervical and pectoral regions (
Penkalski 2001;
Arbour and Currie 2013a). There are usually two cervical half rings composed of an underlying band of non-osteodermal bone and an overlying set of six large osteoderms (
Figs. 6A,
6B); numerous smaller osteoderms are sometimes present surrounding these major osteoderms. The cervical half ring band is made up of coossified, smooth, square, or rectangular segments with varyingly visible sutures in between the segments. Each segment of the band is topped by an osteoderm, and the distal segment on either side is usually enveloped along the distal edge by the osteoderm (
Figs. 6A,
6B).
Carpenter (2004) proposed that
Ankylosaurus had “quarter” rings instead of half rings; i.e., there was a midline gap through the cervical half rings dividing them into two halves. We provide a reinterpretation of the preserved cervical half ring material in AMNH 5895 and argue that these represent more typical “half” rings, without a midline gap.
AMNH 5895 includes two large pieces of cervical half rings that do not join together in any orientation (
Figs. 6C–
6J). Comparison with cervical half rings from
Anodontosaurus and
Euoplocephalus (
Penkalski 2001;
Arbour and Currie 2013a;
Figs. 6A,
6B) revealed that the larger cervical half ring fragment from AMNH 5895 includes the left lateral and distal osteoderms of the first cervical half ring (
Figs. 6C–
6F) and the smaller piece represents the left distal osteoderm from the second cervical half ring (
Figs. 6I,
6J).
Carpenter (2004) suggested that there was no evidence for an underlying bony band in
Ankylosaurus, but the underlying band is visible on both fragments, although it is easier to discern on the fragment of the second cervical half ring. Overall, the cervical half rings of
Ankylosaurus most closely resemble those of
Euoplocephalus and
Anodontosaurus (
Arbour and Currie 2013a), which generally have osteoderms with oval bases and low to moderate keels; they are unlike those of
Scolosaurus, which has circular osteoderms with low conical points (
Arbour and Currie 2013a), or
Ziapelta, which has rectangular osteoderms with tall keels (
Arbour et al. 2014a). They differ even more from the cervical half rings of the more distantly related
Shamosaurus, which has strongly anteriorly directed apices on the distal osteoderms (
Arbour and Currie 2016).
Although the tail of
Ankylosaurus is poorly known, AMNH 5214 includes a portion of the tail club handle and a complete, well-preserved knob (
Fig. 7). The handle vertebrae are twice as wide as those of
Anodontosaurus and
Dyoplosaurus, but are not longer. As such, the tail of
Ankylosaurus may have been shorter relative to overall body length than in
Anodontosaurus,
Scolosaurus,
Dyoplosaurus, or
Zuul, or the tail may have had similar overall proportions but with a smaller tail club. The handle vertebrae of
Ankylosaurus are unique among ankylosaurids, with U-shaped neural spines in dorsal view compared with the V-shaped neural spines in
Anodontosaurus,
Euoplocephalus,
Pinacosaurus,
Talarurus, and most other ankylosaurids (
Maleev 1956;
Maryańska 1969;
Maryańska 1977;
Tumanova 1987;
Carpenter et al. 2011;
Arbour and Currie 2013a;
Loewen et al. 2013). This condition may simply reflect the wider handle of
Ankylosaurus. Although the skull of AMNH 5214 is larger than skulls belonging to
Anodontosaurus or
Euoplocephalus, the tail club knob, at about 45 cm wide (measured from
Carpenter 2004 using ImageJ), is not larger than the largest tail club knobs from Horseshoe Canyon Formation and Dinosaur Park Formation ankylosaurids (
Anodontosaurus specimen AMNH 5425 is 59 cm wide, and an indeterminate tail club from Dinosaur Provincial Park, ROM 788, is 57 cm wide;
Fig. 7). If CMN 8880, which is twice the width of the largest known
Anodontosaurus skull, has a tail club knob twice the size of the largest
Anodontosaurus tail club, then this specimen would have a knob width of 120 cm. This seems exceptionally large, and there must be an upward limit of knob mass that the handle vertebrae can support. Scaling the tail club knob of AMNH 5214 based on the skull width of CMN 8880 yields a knob width of about 57 cm, comparable to that of the largest
Anodontosaurus and
Euoplocephalus knobs.
Newly identified holotype material
Several trays of bone fragments are associated with AMNH 5895, including portions of the skull and cervical half rings that have not been identified or described in previous descriptions of
Ankylosaurus. These include an otic capsule, maxilla fragment, right jugal, left jugal and quadratojugal, two sacral centra, and fragments of the cervical half rings (
Fig. 8).
The maxilla fragment (
Fig. 8A) is 6.3 cm long and 4.2 cm at its widest point. Seven alveoli are arranged diagonally along its long axis, but no teeth are preserved. Based on the angle of the alveoli and the robust projection at one end of the fragment, this might represent the posterior end of the right maxilla.
Parts of the lower portion of each orbit are present (
Figs. 8B,
8C). A fragment representing the left jugal and partial quadratojugal horn (
Fig. 8B) is 14.0 cm long and 13.1 cm high. The horn has a broad base, but the posterior edge is broken, as is the tip, making its overall shape unknown. As in other
Ankylosaurus skulls, postocular caputegulae are absent along the quadratojugal horn. The presence of a thick wedge of plaster infill along the quadratojugal horn suggests that this portion may have been mounted with the rest of the skull at some point. A second fragment representing either the right jugal, right lacrimal, or portions of both (
Fig. 8C) is 10.9 cm long and 5.0 cm high. It is transversely broad and shallowly concave.
An unusual fragment is robust, rounded, and includes a hemispherical concavity with a complex of large pores (
Fig. 8D). This most likely represents one of the otic capsules, but because no other ankylosaur specimens are broken in such a way as to reveal this structure, it is difficult to compare with other species. Its greatest width is 5.2 cm and its greatest length is 6.4 cm. One side of the fragment completely lacks cortical bone surface and reveals a spongy texture. The opposite side includes a circular region approximately 1.3 by 2.3 cm, with four large foramina, and a central, pointed peak.
A poorly preserved fragment of the synsacrum (
Fig. 8H) is 19.7 cm long, and only the ventral surface is preserved. One relatively complete centrum, and approximately one third of a second centrum, are preserved. They are elongate, slightly constricted at the midlength, and possess an indistinct, shallow midline furrow.
Multiple fragments of the cervical half rings (
Figs. 8E–
8G) were identified, although it is not possible to assign individual portions to either the first or second cervical half ring. These represent portions of the cervical half ring band segments which underlie the osteoderms. They have relatively smooth surfaces, with indistinct zigzag sutures marking the boundaries of band segments, and finger-like projections on the anterior and posterior edges.