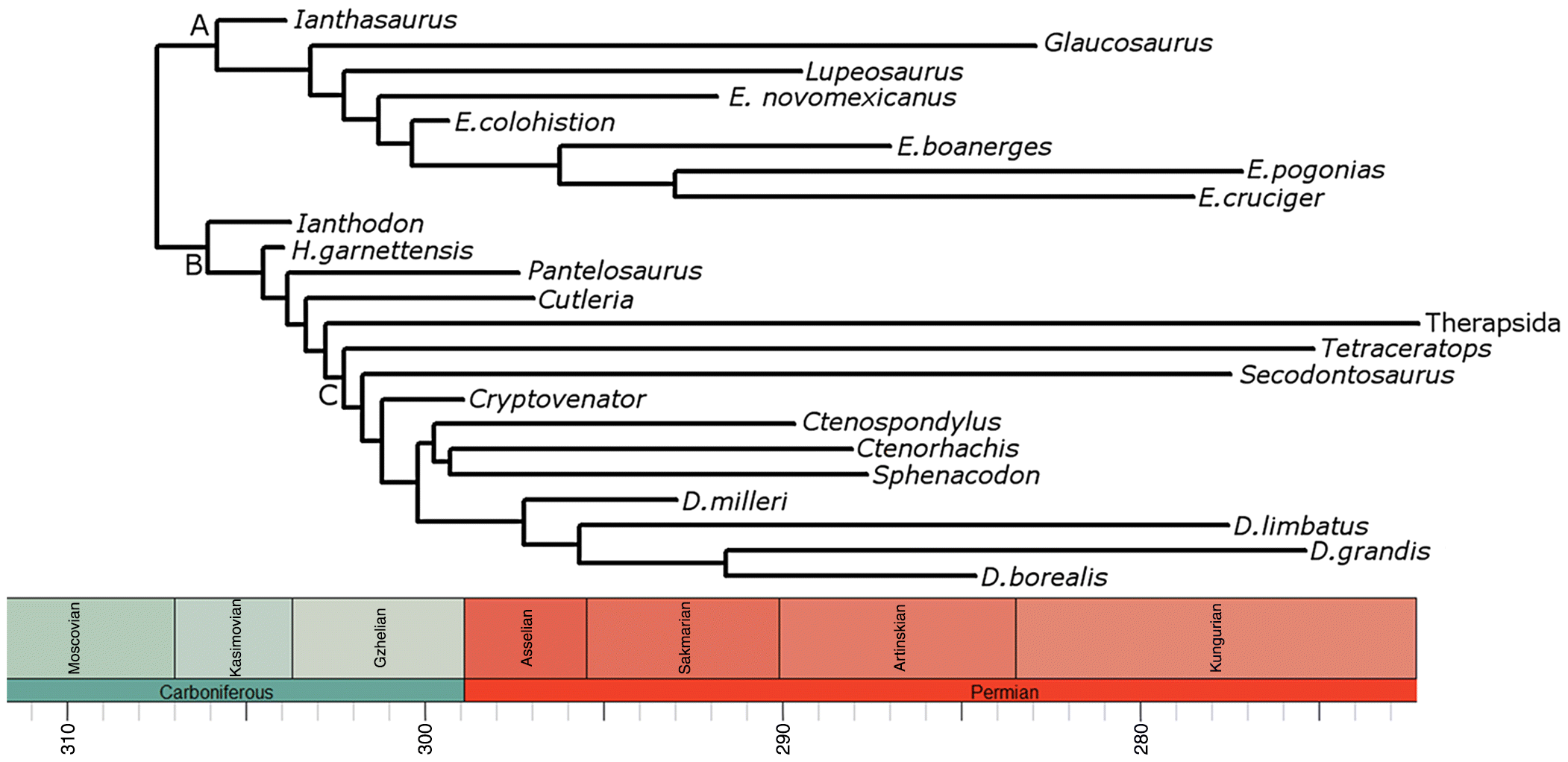
A Carboniferous increase in sphenacomorph body size
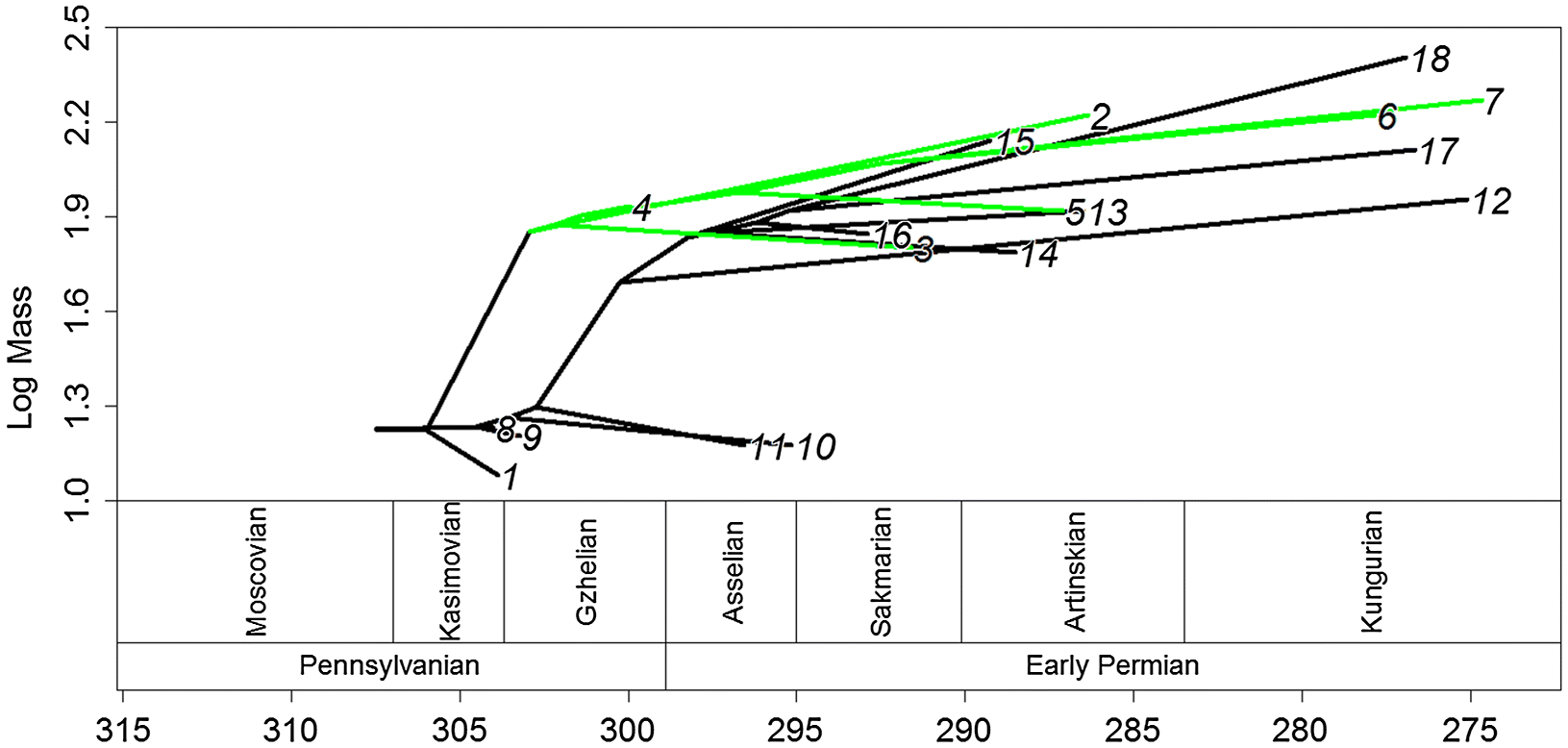
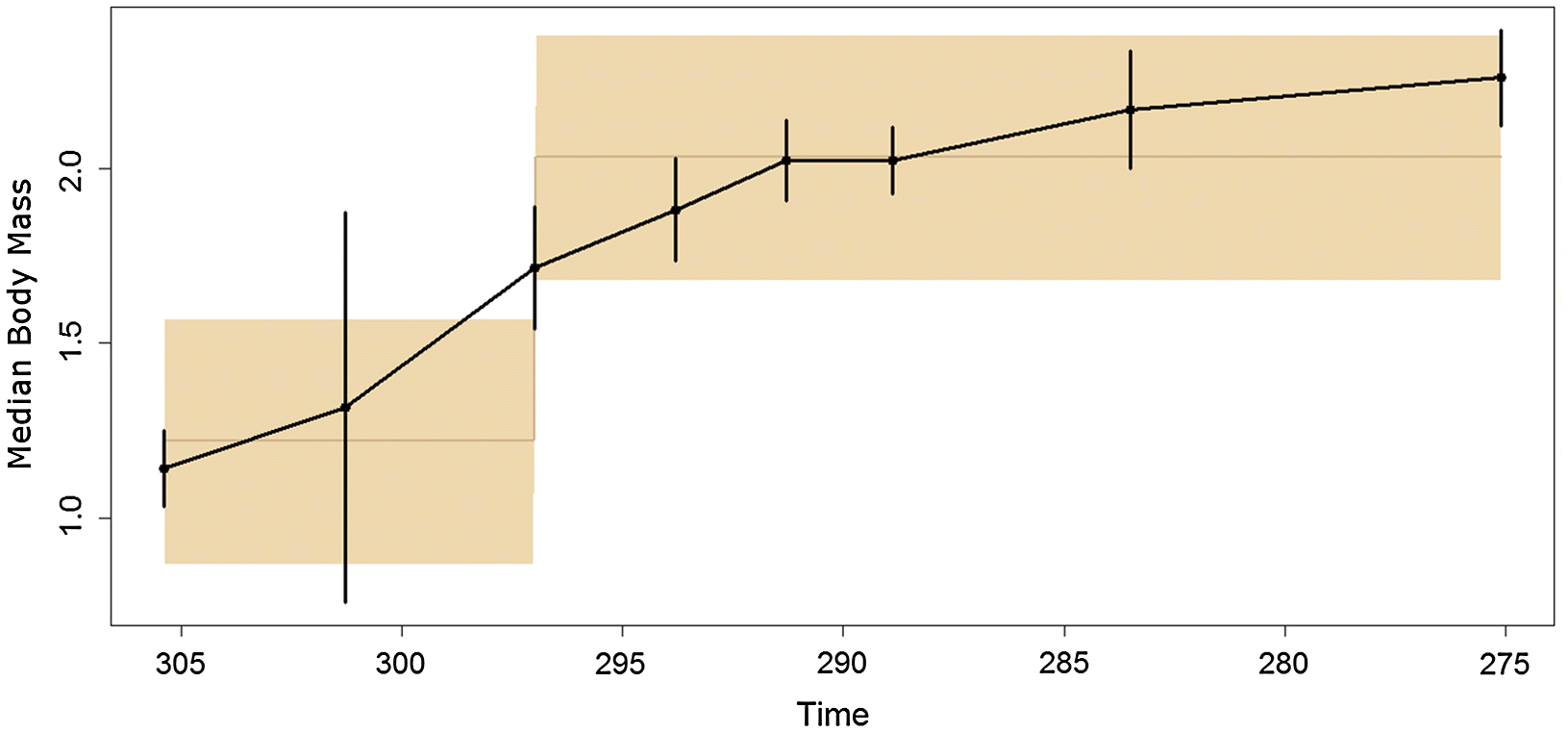
Both of these short, rapid increases in body size occurred during the latest Carboniferous, during the Kazimovian and Gzhelian stage. This observation is supported by the time series of body masses, which showed an increase in mass at this time, followed by a period of relative stability (
Fig. 3). The fitting of evolutionary models to the time series suggests that the punctuated model, with stasis during the Carboniferous and early Permian and a shift to a larger body size in between, best fits the data (although one should again emphasise the weakness of the support for this model). The timing of the shift in body mass is interesting, as it contradicts a suggestion made previously on body mass evolution in pelycosaurian-grade synapsids. In an analysis of changes in species richness of early synapsids (
Brocklehurst et al. 2013), it was tentatively suggested that a rapid trend towards warming and drying during the early Permian at the end of the Sakmarian (
Montanez et al. 2007), which reduced the extent of the equatorial forests, may have promoted the evolution of larger body sizes; a more open environment would allow larger inhabitants (
Brocklehurst et al. 2013). The results here show that this is unlikely to have been the case; although many of the largest pelycosaurian-grade synapsids do not appear in the fossil record until after this event, the initial evolution of large body size appears to have occurred much earlier, during the late Carboniferous. The transition to a more open habitat may have allowed larger taxa to become more abundant, and thus more likely to be preserved (
Brocklehurst and Fröbisch 2014), but it did not precipitate the increase in body size.
The evolution of the dorsal sail showed differing patterns in edaphosaurids and sphenacodontids. The dorsal sail evolved independently in each clade, and evolved more than once in sphenacodontids (once in
Secodontosaurus and once at the base of the
Dimetrodon clade). Sphenacodontids increased in body size before the first occurrence of the dorsal sail in
Secodontosaurus, and species within Sphenacodontidae that do not have extremely elongate neural spines (
Sphenacodon Ctenorhachis and
Ctenospondylus) still evolved large body sizes, comparable to contemporaneous species of
Dimetrodon. This pattern suggests no correlation between the evolution of large body sizes and the development of a dorsal sail in sphenacodontids. However, edaphosaurids increased in body size after the first occurrence of the dorsal sail in
Ianthasaurus, and so it is possible that the two traits are related. Given the differences in patterns between body size and dorsal sail evolution, the differences in neural spine shape and ornamentation, and that thermoregulatory hypotheses for the dorsal sail have been questioned, these results lend support to the hypothesis that elongate neural spines are a secondary sexual character in Sphenacomorpha (
Tomkins et al. 2010). The dorsal sail, along with increased body mass, became more prominent after the decline of rainforests at the end of the Carboniferous (
Sahney et al. 2010), where a dorsal sail would have been less cumbersome.
Phenotypic selection in Edaphosauridae
Selection towards larger body size in edaphosaurids appears to coincide with the evolution of herbivory. Although no skull material from
Lupeosaurus has been preserved, the ribs show curvature throughout their length (
Sumida 1989). This produces the barrel-like chest cavity characteristic of herbivorous pelycosaurian-grade synapsids, thought to be necessitated by the long intestine required for gut fermentation of plant material (
Sumida 1989;
Reisz and Fröbisch 2014).
Edaphosaurus’s status as an herbivore is clear; the leaf-shaped serrated teeth and occluding tooth plates formed from the palatal and dentary teeth are indicative of an herbivorous diet (
Romer and Price 1940;
Modesto 1995;
Sues and Reisz 1998).
The coincidence of the evolution of herbivory and selection towards large body size in edaphosaurids provides support for the observation of
Reisz and Fröbisch (2014) that the earliest terrestrial herbivores showed a tendency towards larger body size, although it should be noted that the universality of this “rule” has been challenged; in captorhinids, the evolution of large size was found to be unrelated to diet (
Brocklehurst 2016). One must also consider the precise reason behind this relationship. In African ungulates, larger body size is correlated with reduced predation; larger taxa have fewer predators and a lower percentage of their mortalities are caused by predation (
Sinclair et al. 2003). However, the increase in body size in the predatory sphenacodontids, the largest and most abundant terrestrial predators of their time, occurred later than the increase in herbivorous edaphosaurids (
Fig. 3). Therefore, increased predation seems an unlikely selective pressure towards larger size. Alternative explanations are physiological. The abundance-packet size hypothesis (
Olsen 2015) suggests that because larger taxa have greater energy requirements, a lineage that evolves a larger body size will experience a selective pressure towards finding a more abundant food source, such as plant matter (or alternatively find food in larger “packets”, i.e., feed on larger prey). However, this explanation does not fit in the case of edaphosaurids. The abundance-packet size hypothesis suggests that the transition to herbivory should occur in larger taxa; that is, the diet transition should follow the change in body size. This hypothesis provides no selective pressure towards larger body size in herbivores, and cannot explain the phenotypic selection identified in this lineage.
A more fitting model linking herbivory and body size is the Jarman-Bell principle (
Geist 1974). This principle posits that although larger animals have larger absolute energy requirements, smaller animals have higher metabolic energy requirements relative to their body size. Therefore, larger herbivores are able to subsist on lower quality plant material such as leaves. Because such low quality plant material is more abundant than higher quality material such as roots and fruits, this model does supply a selective pressure towards larger body size in an herbivorous lineage, and can therefore provide an explanation for the positive phenotypic selection observed in edaphosaurids.
One must consider the small specimen from the Stephanian-aged Rakonitz coal basin (equivalent to Gzhelian) of the Czech Republic, assigned to the species
Edaphosaurus mirabilis. If its assignment to
Edaphosaurus is correct, this would represent the smallest species of this genus, with an estimated mass of 5 kg (
Romer and Price 1940). The assignment of this specimen to
Edaphosaurus is unclear due to its incompleteness (only a single dorsal centrum and partial neural spine is preserved), and it has been suggested that it may belong to
Ianthasaurus (
Modesto and Reisz 1990a), a small insectivorous edaphosaurid from the Carboniferous of the USA (
Reisz and Berman 1986;
Modesto and Reisz 1990b;
Mazierski and Reisz 2010). Nevertheless, even if its assignment to the herbivorous
Edaphosaurus is assumed to be correct, this does not alter the inference of phenotypic selection; when
E. mirabilis is added 100 times to random positions within
Edaphosaurus, positive phenotypic selection towards larger body size is still identified along the same lineage leading to the herbivorous edaphosaurids. Significant phenotypic selection is also identified in
E. mirabilis itself in the majority of these 100 phylogenies, indicating that if its assignment to
Edaphosaurus is correct and it is not a juvenile, it represents an example of miniaturisation. It does not overturn the inference of selection towards larger body size in the herbivores.
Phenotypic selection in Sphenacodontia
The known Carboniferous and early Permian Sphenacodontia form an entirely carnivorous set of taxa. While therapsids are included in the clade Sphenacodontia, and do include herbivorous taxa, they are unknown from the Carboniferous and early Permian fossil record with the exception of a set of sacral vertebrae (
Spindler 2014). As such, therapsid body size evolution cannot be compared with the Sphenacodontidae or Edaphosauridae, and is discounted from this discussion.
As was observed in edaphosaurids, Sphenacodontia showed significant selection towards a larger body size occurring along a single lineage leading to the Sphenacodontidae, the dominant clade of large terrestrial marco-carnivores in the early Permian. This rapid increase in body size occurred shortly after it occurred in Edaphosauridae, and the two may have been linked. It is unsurprising that larger carnivores have been shown to be able to feed on a wider range of prey (
Sinclair et al. 2003). As the appearance of large Edaphosauridae provided a wider range of available prey sizes, there would have been a selective pressure towards larger carnivores able to exploit the greater variety of prey items available.
It should be noted, however, that this explanation does rely on the inference of a predator–prey relationship in the absence of definite evidence. While
Dimetrodon and
Edaphosaurus are often assumed to have existed in a predator–prey relationship (
Romer and Price 1940), there is little actual data for the diet of sphenacodontids in the fossil record (although one notable example is a skeleton of the temnospondyl
Zatrachys in the body cavity of
Dimetrodon milleri (
Romer and Price 1940)). There is a definite disparity in the abundance of sphenacomorphs, with edaphosaurids being considerably less common than their supposed sphenacodontid predators (
Olson 1966). This latter point implies that sphenacodontids must have, at the very least, supplemented their diet with other tetrapods or fish (
Romer and Price 1940;
Olson 1966;
Bakker 2015).
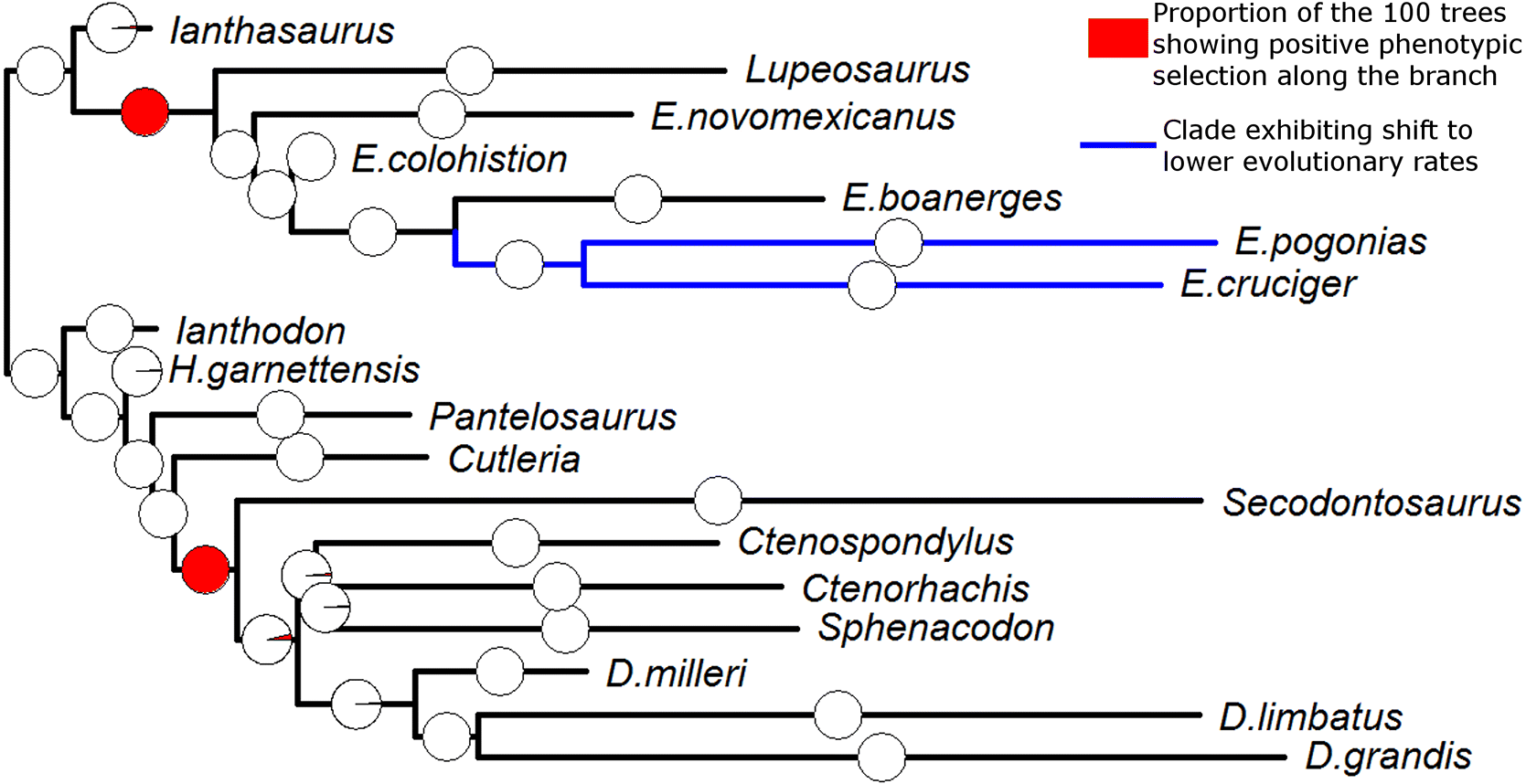
Moreover, the nature of the body size increase in Sphenacodontia is less clear than in Edaphosauridae. While positive phentotypic selection is well supported in all 100 time-calibrated trees containing the taxa subjected to cladistic analysis, when the sphenacodontian taxa not subjected to phylogenetic analysis were added at random to their supposed genera, support for phenotypic selection was only found in 42% of analyses. It appears that the inference of significant selection towards larger body size at the base of Sphenacodontidae is highly dependent on the taxa included in the analysis.
Even if we accept the results of the analyses including the smaller sample of taxa, the magnitude of the increase in size at the base of the Sphenacodontidae is less than that of Edaphosauridae. A further difference between the two can be observed in the period following these two rapid increases. While the carnivorous Sphenacodontidae continued to increase in size throughout the early Permian, the herbivorous members of Edaphosauridae showed little change in body mass following their initial increase (
Fig. 3). The results of the TM1 analysis support this observation; a shift towards lower rates of size evolution was identified at the node containing the largest species of
Edaphosaurus (
Fig. 2), which presumably have converged on this adaptive optimum.
This last contrast allows us to infer differing modes of phenotypic selection acting on the high-fibre herbivores and the macro-carnivores. While the lineage leading to herbivores does exhibit selection towards a mass larger than that of the ancestral taxa, this appears to be selection towards an adaptive optimum rather than a constant trend. Once this optimum is reached, there is a shift towards lower rates of evolution and body sizes remain within a relatively narrow range. On the other hand, the sphenacodontid top-predators continued to evolve ever-larger body sizes throughout the early Permian. By the Kungurian, the lineage leading to
Dimetrodon grandis had exceeded the largest known edaphosaurid. The increased diversity and abundance of some of the largest terrestrial animals of the early Permian, the caseids, in the latter stages of the Cisuralian (
Olson 1954,
1968;
Reisz et al. 2011;
Brocklehurst et al. 2013;
Romano and Nicosia 2014) may provide a reason for the continued increase in body size: the need to expand the possible diet range to include these even larger prey items. The sphenacodontids also evolved a more sophisticated dental apparatus at this time: “true” ziphodonty, where the serrations of the teeth have denticles with a dentine core (
Brink and Reisz 2014).