Introduction
The Poweshiek skipperling (
Oarisma poweshiek Parker, 1870) is a small, orange-brown butterfly (Lepidoptera: Hesperiidae) that occurs in isolated populations within its North American range. Historically occurring in eight states and one province, this species is now believed to be restricted to only two jurisdictions: a few isolated prairie fens in Michigan and several areas of tall grass prairie in Manitoba (
COSEWIC 2014;
Delphey et al. 2016;
Belitz et al. 2020). The restricted distribution of Poweshiek skipperling is a result of an alarming decline that has occurred over the past few decades (reviewed in
Swengel and Swengel 2014;
Belitz et al. 2020), prompting several jurisdictions to protect the species. In Canada, the Poweshiek skipperling is listed as endangered and protected through both federal and provincial species-at-risk legislation (
Environment Canada 2012;
Government of Manitoba 2020). This population's isolation from natural rescue populations, its small size and ongoing decline, and its restricted distribution make it vulnerable to stochastic events and extirpation (
COSEWIC 2014).
Poweshiek skipperling are prairie specialists that rely on native wet-mesic and dry prairie habitat (
Catling and Lafontaine 1986;
Selby 2005), with adults feeding on forb species primarily associated with dry prairie and larvae feeding on graminoid species primarily associated with wet prairie (
Henault and Westwood 2019). They are rarely found in non-native vegetation (
Swengel and Swengel 1999;
Henault and Westwood 2022;
Henault and Westwood 2023).
Catling and Lafontaine (1986) first documented the species in Canada in 1985, when they were locally abundant in native tall grass prairies near Vita, Manitoba. Prior to cultivation in the 1800s, there was at least 6000 km
2 of tall grass prairie in Manitoba (
Samson and Knopf 1994;
Henderson and Koper 2014). Through conversion to annual cropland and urban development, native tall grass prairie is now all but gone from Manitoba, with only a few areas remaining, much of which are within the 44.5 km
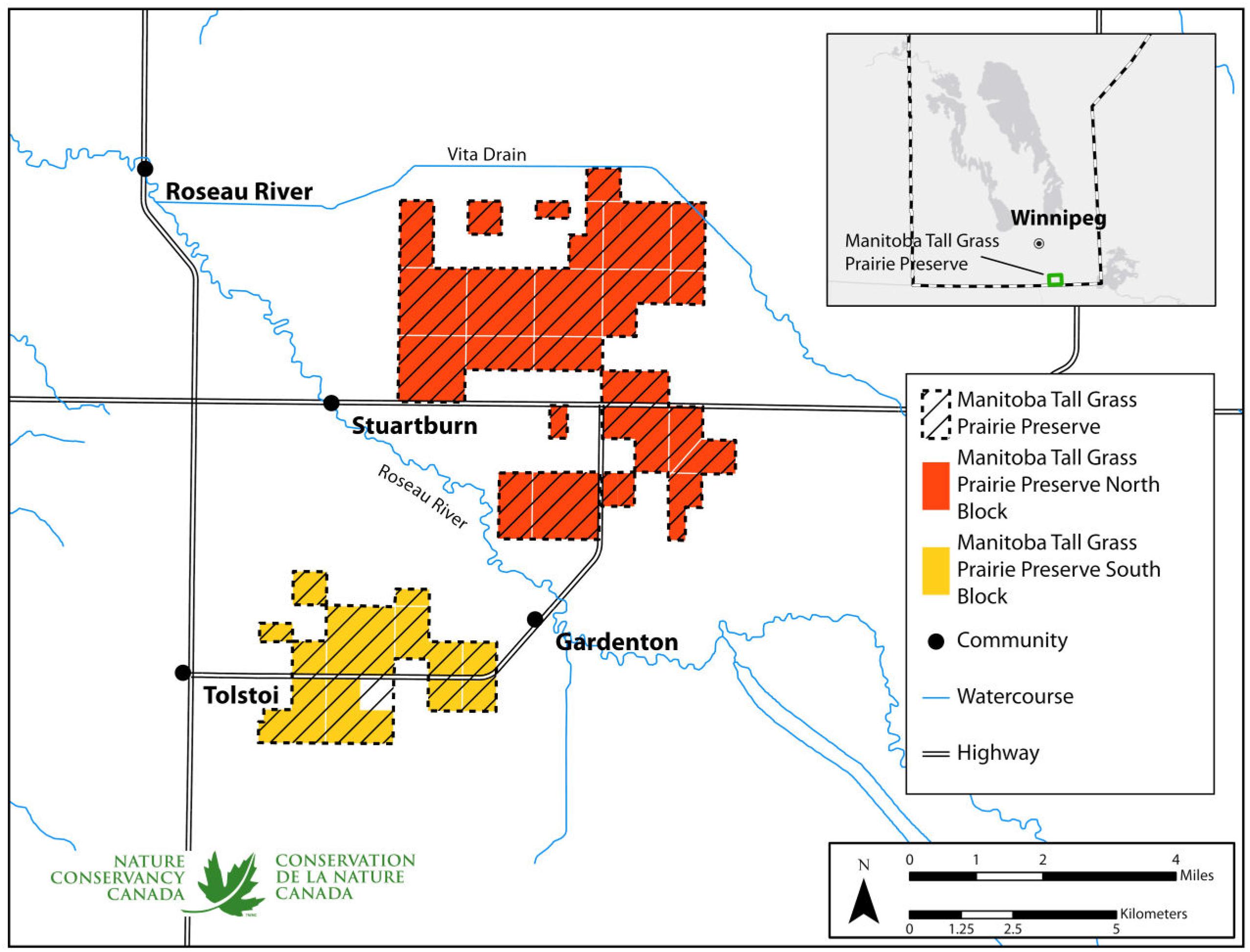
2 (as of 2023) Tall Grass Prairie Preserve (TGPP) and surrounding localities in southeastern Manitoba (
Fig. 1). Established in 1989 through efforts by the Critical Wildlife Habitat Program (a cooperative initiative involving Nature Manitoba, World Wildlife Fund, Wildlife Habitat Canada, Manitoba Habitat Conservancy (formerly Manitoba Habitat Heritage Corporation), and Manitoba Natural Resources and Northern Development (formerly Manitoba Conservation and Climate), the TGPP encompasses large and contiguous blocks of endangered tall grass prairie (
Grantham et al. 2021). The TGPP is part of the larger Tallgrass Aspen Parkland international conservation landscape that extends from near Red Lake Falls, Minnesota to Steinbach, Manitoba, extending over 2,170,000 acres (878,000 ha), an area identified as one of only six “very high conservation priority” sites in the Northern Tallgrass Prairie Ecoregional Plan (
Northern Tallgrass Prairie Ecoregional Planning Team 1998). The Canadian population of Poweshiek skipperling is restricted to this area (TGPP and surrounding localities) of tall grass prairie. There has been relatively little habitat loss in the TGPP and surrounding areas in recent decades (
COSEWIC 2014), though many regional tall grass prairie patches are declining in size due to woody species encroachment (
Koper et al. 2010). It is unknown how local conditions, such as changing climate, may have altered the suitability of the tall grass habitat for the population over this time.
The Poweshiek skipperling has one generation per year (univoltine) and does not migrate (
Belitz et al. 2019;
Henault and Westwood 2023). The timing of life cycle stages varies across the species’ range and between years depending on weather (
Selby 2005). In Manitoba, the brief adult flight period occurs from late June to late July, during which time eggs are laid on host plant leaves (
Henault and Westwood 2019;
Henault and Westwood 2022). Host plants in Manitoba include grasses such as big bluestem (
Andropogon gerardii Vitman), mat muhly (
Muhlenbergia richardsonis (Trin.) Rydb)
, prairie dropseed (
Sporobolus heterolepis (A. Gray) A. Gray), and little bluestem (
Schizachyrium scoparium (Michs.) Nash) (
Dupont Morozoff 2013;
Henault and Westwood 2022). Larvae feed on host plants throughout the remainder of the summer and enter a form of dormancy as larvae in mid-to-late September (K. Eckhardt (personal communication, 24 October 2023)), overwintering at the base of host plant species in or above the litter layer (
Borkin 1995;
Selby 2005;
Henault and Westwood 2022). Larvae emerge from dormancy in May and resume feeding, reaching at least the sixth instar before pupating in mid-to-late June (
McAlpine 1973; K. Eckhardt (personal communication, 24 October 2023)). Black-eyed Susan (
Rudbeckia hirta L.), pale-spiked lobelia (
Lobelia spicata Lam.), and upland white aster (
Solidago ptarmicoides (Torr. & A. Gray) B. Boivin) have been the primary nectar plants of adults in Manitoba (
Catling and Lafontaine 1986;
Semmler 2010;
Dupont Morozoff 2013;
Westwood et al. 2020), though Poweshiek skipperling have recently been observed nectaring on other species (
Henault 2021).
Catling and Lafontaine (1986) observed the highest Poweshiek skipperling densities in areas with high pale-spiked lobelia densities, but pale-spiked lobelia was not present during
Semmler (2010) and
Dupont Morozoff's (2013) studies and was also scarce in
Henault's (2021) study. In Michigan, Poweshiek skipperling are more likely to be found in areas with high numbers of their preferred nectar plants (
Belitz et al. 2019). Adults have poor dispersal ability, with maximum distances estimated at only 1–1.6 km (
Burke et al. 2011) and are unlikely to cross physical barriers such as roads and tall vegetation in their fragmented landscape (
Westwood et al. 2012). In Michigan, obstructive vegetation at only 1.5 m tall acted as a barrier, reducing the likelihood of the presence of Poweshiek skipperling in otherwise suitable areas (
Belitz et al. 2019). Individuals observed in Manitoba rarely travelled more than 20–30 m beyond the boundaries of occupied sites, even when suitable sites were available nearby (
Dupont Morozoff 2013; M. Olynyk (unpublished data)).
The cause(s) of the recent rapid Poweshiek skipperling population decline in Manitoba are not well understood. Previous range contractions in the United States have been attributed to inadequate or inappropriate management resulting in unsuitable habitat (
Swengel et al. 2011), though there are mixed results on the impacts of management (e.g., prescribed burning and grazing) on Poweshiek skipperling abundance (
Royer and Marrone 1992;
Swengel 1998;
Swengel and Swengel 1999;
Dupont-Morozoff et al. 2022). Prior to European colonization, the tall grass prairie was maintained by periodic wildfires and bison (
Bison bison bison Linnaeus, 1758) grazing that suppressed ecological succession to shrubby habitat and reduced plant litter (
Knapp et al. 1999;
Allen and Palmer 2011). Therefore, management methods that approximate these natural disturbances, such as prescribed burning, cattle grazing at appropriate intensities, and mechanical brush control, have become important tools for maintaining tall grass prairie. Lack of land management can lead to natural woody encroachment and invasion by non-native plant species, which can reduce host and nectar plant availability (
Dornbush 2004). However, these practices may result in direct butterfly mortality, and responses to prescribed burning and grazing vary among butterfly species (
Vogel et al. 2007). For prescribed burning, a species’ response can be unpredictable, depending on the mortality rate from the burn and the ability of individuals to disperse from adjacent untreated sites to recolonize the burned site (
Swengel and Swengel 1999;
Swengel and Swengel 2014). For Poweshiek skipperling, there are several limiting factors (e.g., isolated population, limited dispersal ability, a single generation per year) that make them particularly susceptible to excessive prescribed burning (e.g., burning that is too frequent, poorly timed, or too intense) (
Swengel et al. 2011) because these factors are associated with slow population recolonization and recovery if the population is reduced (
Swengel 1996;
Panzer 2002). Altered floral composition within burned areas (
Howe 1995;
Towne and Kemp 2008) may also result in several years before the floral community is suitable to skipperling again (
Swengel 1996;
Dupont Morozoff 2013). Previous studies have shown it typically takes 2–5 years for Poweshiek skipperling and other butterflies to recover post-burn (
Swengel 1996;
Panzer 2002), but some species were not fully recovered after 5 years (
Vogel et al. 2010). The sensitivity of Poweshiek skipperling to burns (
Swengel 1996;
Webster 2003) therefore warrants a thoughtful approach. Having a nearby site of core habitat for refuge is necessary (
Swengel and Swengel 2007), and burning less than 20% of the occupied area has been recommended for Poweshiek skipperling and other butterflies (
Swengel 1996). In this way, burning small patches to create a mosaic of habitat can benefit poor dispersers such as the Poweshiek skipperling, which may have difficulty recolonizing large, burned areas (
New et al. 2010).
Another practice that can be used to maintain prairie habitat in its native, early successional state for Poweshiek skipperling is grazing by domestic cattle (
Bos taurus Linnaeus, 1758). Grazing is generally less disruptive to butterflies than burning because it does not reduce entire stands of vegetation or remove the litter layer in which butterfly larvae reside (
Swengel 1996). However, cattle grazing can have different effects on prairie structure than bison grazing, which historically maintained the tall grass prairie (
Knapp et al. 1999). For example, bison grazing has been shown to result in higher species richness, higher spatial heterogeneity, higher herbaceous biomass, reduced bare ground, and fewer invasive species than cattle grazing (
Towne et al. 2005;
Hillenbrand et al. 2019;
Ratajczak et al. 2022). These impacts are all important for maintaining ecosystem function; however, cattle grazing is much more practical in most cases, and grazing by either species at low to moderate stocking rates is more beneficial for maintaining prairie habitat and species richness than not grazing at all (
Towne et al. 2005;
Ratajczak et al. 2022). The risk of trampling or consumption of adults, eggs, and larvae is presumably low unless stocking rates are high (
Dupont-Morozoff et al. 2022; as seen for bird nests in
Bleho et al. 2014). High stocking rates also result in unselective heavy grazing that removes host and nectar plants and severely diminishes the quality and diversity of tall grass prairie (
Howe 1994;
Swengel 2008). On the other hand, conservatively grazed prairie may have greater forb cover and species diversity because grazing removes dominant grass cover (
Vinton et al. 1993;
Towne et al. 2005).
Extreme weather is another factor that may have influenced Manitoba Poweshiek skipperling abundance, especially because small populations are more vulnerable to stochastic weather events. Extreme weather relative to species’ tolerances predicts species abundance and local extinction risk in insects and terrestrial vertebrates (
Kerr 2020;
Soroye et al. 2020;
Williams and Newbold 2021). For butterflies specifically, weather has been shown to be a strong determinant of population dynamics (
Roy et al. 2001;
Woods et al. 2014). Univoltine butterflies in the United Kingdom are most vulnerable to extreme heat during the winter, extreme cold during the adult life stage, and extreme precipitation as pupae (
McDermott Long et al. 2017). These effects were more significant for habitat generalists than for habitat specialists. For species overwintering in cold climates, the ground temperature is moderated from extreme temperature fluctuations by the consistent snowpack (
Geiger 1965;
Sharratt et al. 1992), but extreme heat in the winter can melt this snowpack and make overwintering butterflies vulnerable to fluctuating temperatures, reducing their survival (
Scriber et al. 2012). In addition, butterflies have appeared in spring significantly earlier over the past several decades due to warmer spring weather (
Roy and Sparks 2000;
Forister and Shapiro 2003). This may be concerning if it results in a timing mismatch with a population's obligate host plant(s), such as that occurred in Indiana when larvae of the endangered Karner blue butterfly (
Lycaeides melissa samuelis Nabokov, 1944) hatched early and before its host plant, wild blue lupine (
Lupinus perennis L.), became available, resulting in low larval survival (
Patterson et al. 2020). Poweshiek skipperling may also be vulnerable to extreme weather outside of their physiological tolerances that has been exacerbated by climate change, such as loss of snow cover in the winter or a cool, wet spring (
Selby 2005). Because the Manitoba tall grass prairie is susceptible to flooding due to naturally poor drainage and a high water table in the area (
Westwood et al. 2020), increased precipitation may negatively impact this population. In their analysis of wide-range Poweshiek skipperling populations over time,
Belitz et al. (2020) concluded that weather drove presence prior to the steep population declines that began in the early 2000s, but since these steep declines, presence has been driven primarily by land cover (a positive effect of natural land cover and a negative effect of fragmentation). Even so, increased temperatures were negatively correlated with the probability of Poweshiek skipperling presence during the recent declines (
Belitz et al. 2020). Precipitation extremes did not appear to be an important factor explaining the probability of presence during the declines. Understanding the effects of extreme weather specifically on the Poweshiek skipperling population in Manitoba since its steep decline began may enable land managers to predict population fluctuations and adjust management activities accordingly.
At the northern edge of the Poweshiek skipperling range, in the tall grass prairie of Manitoba, there is still much to learn about the population dynamics of this endangered species. The Nature Conservancy of Canada (NCC) has co-managed the TGPP, where these butterflies are found, with several partners since the 1990s and annual Poweshiek skipperling inventory and monitoring have consistently occurred since 2009. Using a decade of survey data, our objectives were to assess how various habitat characteristics, land management practices, and extreme weather affected the Poweshiek skipperling population in Manitoba. Specifically, we were interested in (1) which habitat characteristics (black-eyed Susan density and distance to the nearest occupied site) were associated with higher Poweshiek skipperling abundance, (2) whether the timing since broad-scale management practices (prescribed burning and grazing) last occurred correlated with higher Poweshiek skipperling abundance, and (3) the relationship between extreme weather (temperature or precipitation) during winter–spring (larvae–pupae) and Poweshiek skipperling abundance (adult butterflies) in the summer.
Discussion
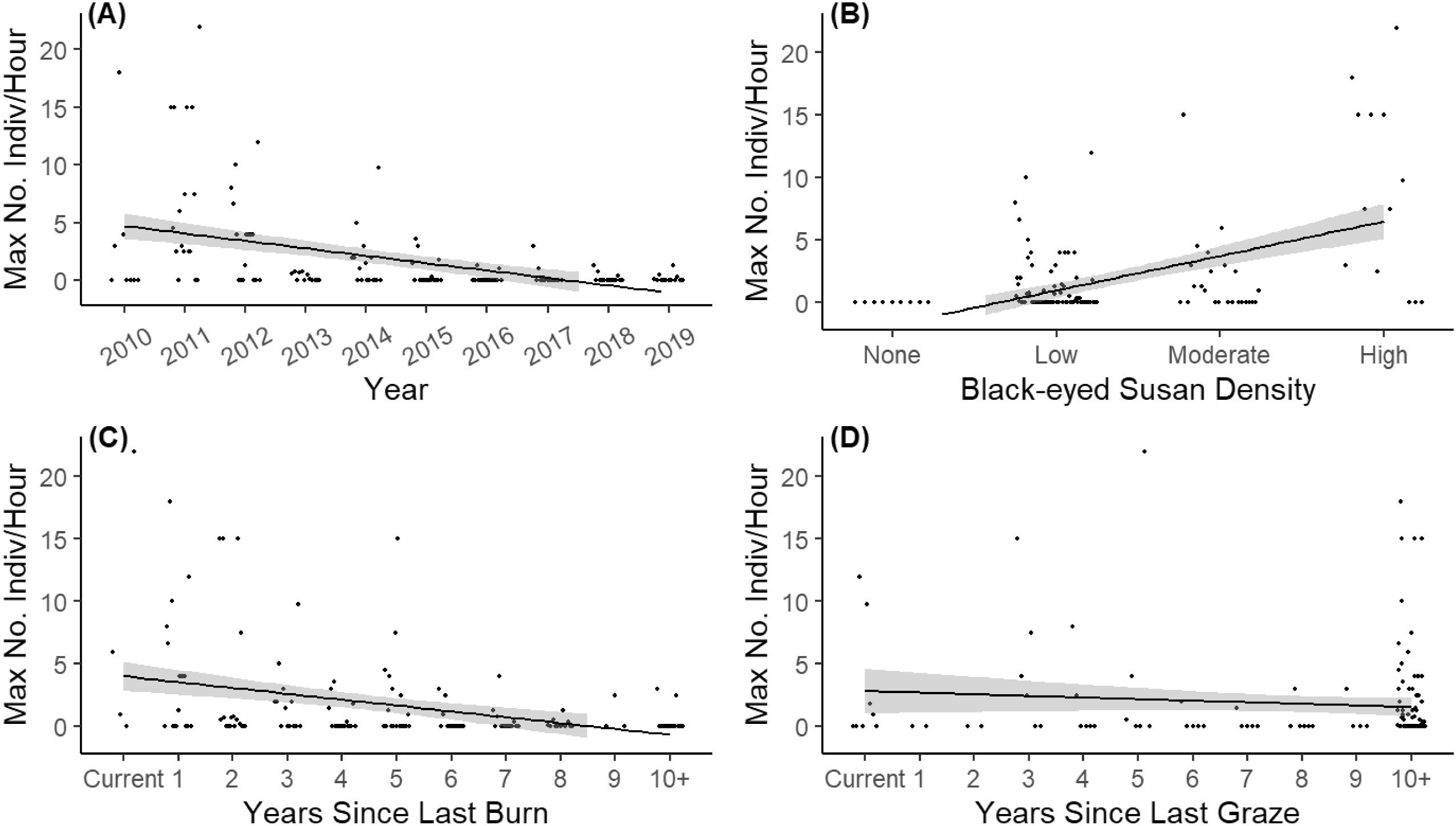
The endangered Poweshiek skipperling has experienced precipitous declines in Manitoba over the past two decades. Understanding the factors that influenced their abundance over ten of these years is essential for land managers to effectively support the recovery and long-term persistence of this population through thoughtful land management practices. We found that black-eyed Susan density was the variable with the largest effect on Poweshiek skipperling abundance, being positively correlated (
Table 2). Furthermore, Poweshiek skipperling were never present in sites with no black-eyed Susans (
Fig. 2B). This plant appears to be an important nectar source for adult Poweshiek skipperling in Manitoba, though they will also visit other species if in bloom during the adult flight period (
Semmler 2010;
Dupont Morozoff 2013;
Henault and Westwood 2023). As nectar is the adult Poweshiek skipperling's only food source, it follows that management actions ensuring black-eyed Susan and other primary nectar plants are present in high densities are important for maintaining high Poweshiek skipperling abundances within sites. These results contrast with a recent analysis of data from the TGPP spanning 2008–2009, which found no correlation between black-eyed Susan density and Poweshiek skipperling density (
Dupont-Morozoff et al. 2022). These different results may be explained by the increased precision in the 2008–2009 black-eyed Susan measurements, where actual counts were made per unit area versus visual estimates of density. On the other hand, there were only 11 sites in this 2-year analysis, and black-eyed Susan density was relatively high in all sites.
Habitat configuration at the landscape scale was also important for Poweshiek skipperling, as indicated by their increased abundance when closer to another occupied site. This is not surprising given that these butterflies are considered poor dispersers (
Burke et al. 2011). Ongoing habitat loss and fragmentation in this area may threaten this species, as we found that 17% of the nearest occupied sites were further than 1.6 km, the maximum estimated dispersal distance for Poweshiek skipperling (
Burke et al. 2011). In fact,
Dupont Morozoff (2013) observed that individuals in Manitoba rarely travelled more than 20 m beyond the boundaries of inhabited sites, even when suitable sites were available nearby, suggesting that even small amounts of habitat fragmentation might threaten this species. Skipperling also actively avoid treed areas adjacent to tall grass prairie sites (
Dupont Morozoff 2013;
Henault and Westwood 2023), suggesting that succession towards forest habitats in this region leads to poor landscape permeability. Forested areas have been observed to act as barriers to movement of other prairie butterfly species (
Stasek et al. 2008); in Michigan, 1.5 m tall vegetation acted as a barrier and reduced the likelihood of Poweshiek skipperling presence in otherwise suitable areas (
Belitz et al. 2019). These results suggest that Poweshiek skipperling in Manitoba may have difficulty recolonizing sites once locally extirpated.
Three of the four extreme weather variables (degree days above the lower threshold during the pre-active period, lower threshold degree days during the active period, and cumulative precipitation during the active period) were statistically significant predictors of adult Poweshiek skipperling abundance. Degree days above the lower threshold during the pre-active period had the largest effect on Poweshiek skipperling, being positively correlated with adult abundance. During this period (March–April in Manitoba), Poweshiek skipperling larvae do not experience climate conditions to support resumption of development from dormancy, which most likely starts again in May (K. Eckhardt (personal communication, 24 October 2023)). This positive effect could indicate that warmer spring days were beneficial, possibly because these larvae were able to emerge earlier (
Selby 2005), allowing them more time to feed on host plants and grow before pupating or experiencing less harsh environmental conditions while waiting for new host plant tissue to emerge. There was also a large positive effect of lower threshold degree days (i.e., cool weather) during the active period (May–June, when skipperling are larvae or pupae) on adult Poweshiek skipperling abundance. This was surprising, as cool weather below the Poweshiek skipperling's developmental threshold was expected to have the opposite, negative effect on adult abundance. Consistent with predictions from the literature (
Selby 2005;
McDermott Long et al. 2017), there was a negative effect of precipitation during the active period on adult abundance. Given the Manitoba tall grass prairie is susceptible to flooding (
Westwood et al. 2020), high precipitation may have led to direct mortality of Poweshiek skipperling larvae and pupae by drowning or indirect mortality of larvae by starvation from limited access to host plants. Inconsistent with the literature (
Selby 2005;
McDermott Long et al. 2017), there was no observed effect of thaw degree days on overwintering larvae. This may have been because the thaws were not warm or long enough to actually melt the snowpack.
Two common management practices for maintaining tall grass prairies are prescribed burning and cattle grazing. We found a negative effect of both years since the last burn and years since the last graze on Poweshiek skipperling abundance, indicating that these practices can be beneficial to the Poweshiek skipperling population in Manitoba. The highest Poweshiek skipperling abundances were recorded at sites that had been burned 5 or fewer years prior (
Fig. 2C). This is consistent with results from
Dupont-Morozoff et al. (2022), who found that sites that were burned more than 15 years prior had the fewest Poweshiek skipperling compared to more recent burns (1–6 years). For grazing, there were high skipperling abundances recorded at sites that were grazed 5 or fewer years prior, but also at many sites that were grazed ten or more years prior (
Fig. 2D). As has been suggested in previous studies on this species and others (
Swengel 1996;
Panzer 2002;
Vogel et al. 2010;
Dupont-Morozoff et al. 2022), approximately 5-year burn and grazing cycles appear to be optimal, though Poweshiek skipperling were relatively abundant even 1–2 years after a burn (
Figs. 2C and
2D). Despite these results, caution should still be taken when burning and grazing are implemented by land managers. Burning of small patches (a few hectares or less), interspersed with refuge habitat, may be the best way to ensure the persistence of locally restricted populations that are poor dispersers (
New et al. 2010), such as the Poweshiek skipperling (
Swengel and Swengel 2007). As for reestablishment to occur, burned sites must be within dispersing distance of other occupied sites (
Swengel et al. 2011). As for grazing, cattle stocking rates should be kept low to minimize the risk of trampling and consumption of skipperling or their food plants by cattle. Other mechanisms of habitat disturbance, such as haying and mowing, may be a viable alternative to burning or grazing but have not been studied for Poweshiek skipperling. Haying and mowing have been beneficial for the Dakota skipper (
Hesperia dacotae) in central Manitoba (
Webster 2003;
Rigney 2013).
Acknowledgements
Funding for this research was generously provided by C.P. Loewen Family Foundation Inc., Donner Canadian Foundation, Government of Canada: Environment and Climate Change Canada, Government of Manitoba, Richardson Foundation Inc., Weston Family Foundation, and the hundreds of individual Nature Conservancy of Canada donors. We would like to thank the following people for their contributions over the years to fieldwork, data collection and analysis, Geographic Information System work, and compiling and grazing histories: H. Carrey, L. Reeves, A. Grottoli, A. Westphal, A. Safruk, E. de Greef, K. Eckhardt, L. Burns, P. Des Brisay, R. Kerbrat, M. Balcaen, J. Henault, C. Breiter, C. Savage, A. Papineau, K. Johnson, L. Newediuk, S. Semmler, M. Olynyk, D. Simard, J. Leach, S. Petersen, M. Lalonde, C. Olson, M. Russel, M. Kirbyson, S. Sheard, J. Tkachuk, T. Teetaert, S. Gietz, and J. Becker. We would also like to thank the two anonymous reviewers for reviewing draft manuscripts and providing much valued feedback.