Introduction
The establishment of marine protected areas (MPAs) is a key conservation action used to support and maintain resilient marine ecosystems in the face of climate change (
Magris et al. 2014;
Roberts et al. 2017;
Tittensor et al. 2019;
Jacquemont et al. 2022). Several recent reviews have found that highly protected MPAs will provide the greatest ecological benefits and better buffer against some climate change impacts by alleviating stressors from other human pressures (
Edgar et al. 2014;
Grorud-Colvert et al. 2021;
Jacquemont et al. 2022). MPAs also build resilience against climate impacts by protecting carbon storage and sequestering habitats (e.g., eelgrass, surfgrass, and kelp forests, seabed), promoting genetic diversity associated with higher capacity for adaptation, and restoring food web structure, including the presence of apex predators that may buffer climate-induced instabilities (reviewed in
Roberts et al. 2017). However, despite the value of MPAs for maintaining ocean ecosystem resilience, climate change is a pervasive threat that has and will continue to impact marine ecosystems despite spatial protection measures (e.g.,
Selig et al. 2012;
Ross et al. 2020;
Stevenson et al. 2020), and strong emissions reductions are urgently needed to protect ocean ecosystems (
Bruno et al. 2018;
Bates et al. 2019). As mitigation tools, MPAs and MPA networks help buffer climate change effects on ocean ecosystems (
Micheli et al. 2012;
Olds et al. 2014;
Ziegler et al. 2023); however, there are few examples of MPA networks that have built climate change considerations into their design, and most of them are from tropical ecosystems (
Tittensor et al. 2019).
MPA networks, collections of individual MPAs that operate cooperatively and synergistically at various spatial scales under a range of protection levels (
WCPA/IUCN 2007), offer greater resilience to climate change and other stressors than individual MPAs due to key network design principles including representativity, replication, and connectivity (
Micheli et al. 2012;
Green et al. 2014;
Carr et al. 2017). Standard analytical approaches for incorporating representativity and replication into MPA networks are well established, but incorporating connectivity remains a challenge (
Balbar and Metaxas 2019). One key barrier to incorporating connectivity into MPA design is that it varies with species life history, dispersal ability, habitat associations, and migration patterns, among other ecological traits (
Bryan-Brown et al. 2017). Despite its recognized importance for MPA network effectiveness, a recent review found that only 11% of global MPAs considered connectivity in their design, mostly due to limited data (
Balbar and Metaxas 2019). In the absence of connectivity models for all focal species in the planning process, MPA networks are often designed using rules of thumb to incorporate connectivity. These rules are usually informed by life history characteristics such as planktonic larval duration of focal species (e.g.,
Burt et al. 2014;
D'Aloia et al. 2017) and inform MPA size and spacing recommendations that serve as a surrogate for connectivity in the design of the network.
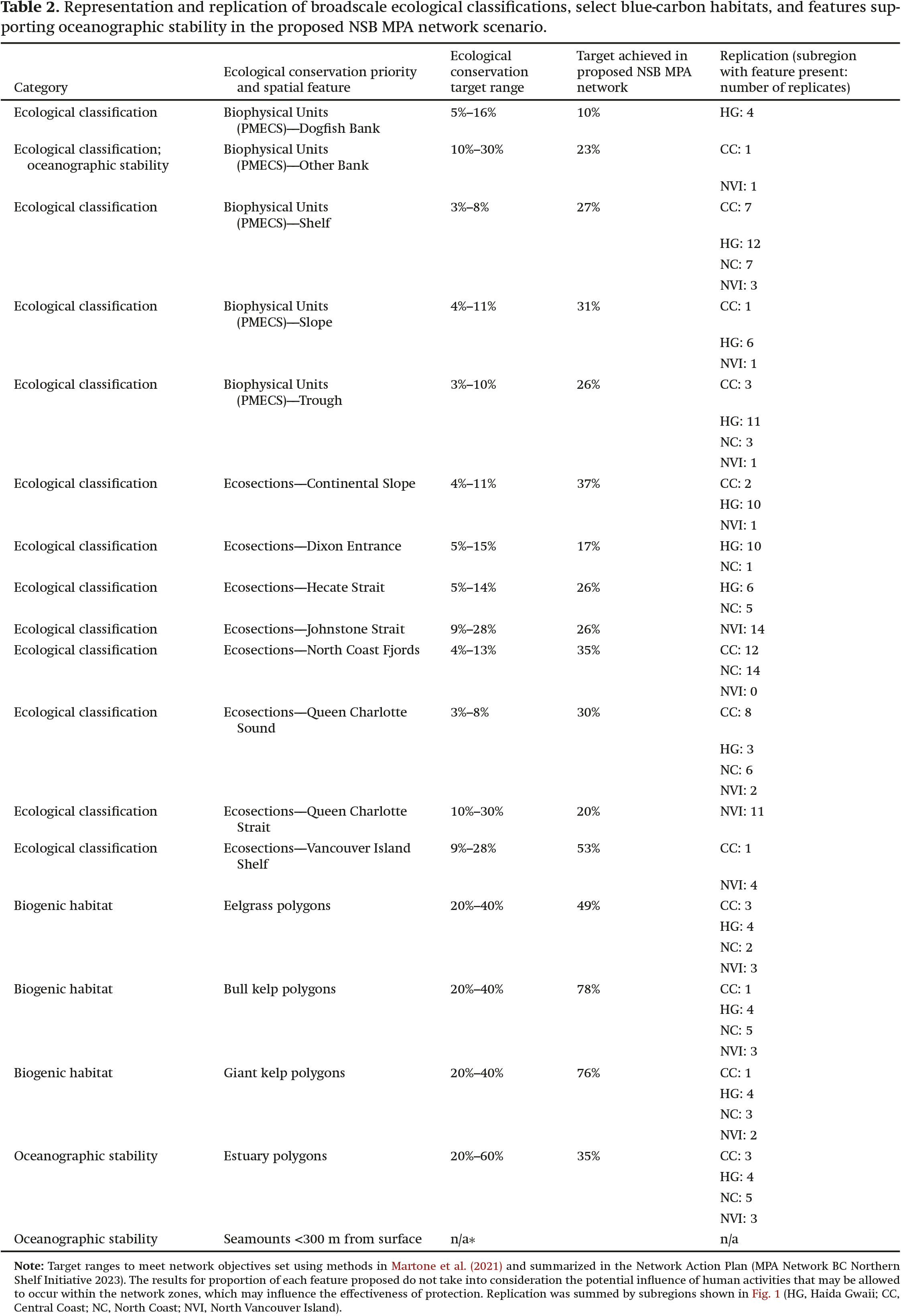
An MPA network that is representative and well-distributed in space and with adequate replication across environmental, physical, and latitudinal gradients allows for potential future shifts in species distributions and is more likely to incorporate areas with different climatic trajectories and histories. These features add further robustness to the MPA network in the face of climate change (
Tittensor et al. 2019). Climate refugia, areas that are more stable or experience incremental change through time (
Barnosky 2008;
Ban et al. 2016), although often rare, have also been recommended for inclusion in protected area design (
Morelli et al. 2016;
Tittensor et al. 2019;
Walsworth et al. 2019;
Wilson et al. 2020). Slower rates of change within climate refugia may allow the time necessary for populations to adapt to changing conditions in comparison to areas that may experience accelerated change. This kind of adaptation to climate change can help determine the persistence of future populations and the magnitude of range shifts (e.g.,
Pinsky et al. 2013). The nature of local adaptation to a range of representative environments in the planning area that includes different climatic histories and trajectories can help to protect the high levels of adaptive genetic variation that are essential to future climate adaptation (
Bell and Gonzalez 2009;
Norberg et al. 2012;
Orr and Unckless 2014;
Thompson and Fronhofer 2019). In the absence of data on the climate histories and genetic diversity of species in the MPA network planning area, an alternative is to rely on representativity to ensure that a range of physical (e.g., depth), geographical (e.g., latitude), and environmental (e.g., temperature) gradients are well represented, replicated, and adequately connected in the MPA network, thereby providing the greatest chance that climate and genetic heterogeneity are also included.
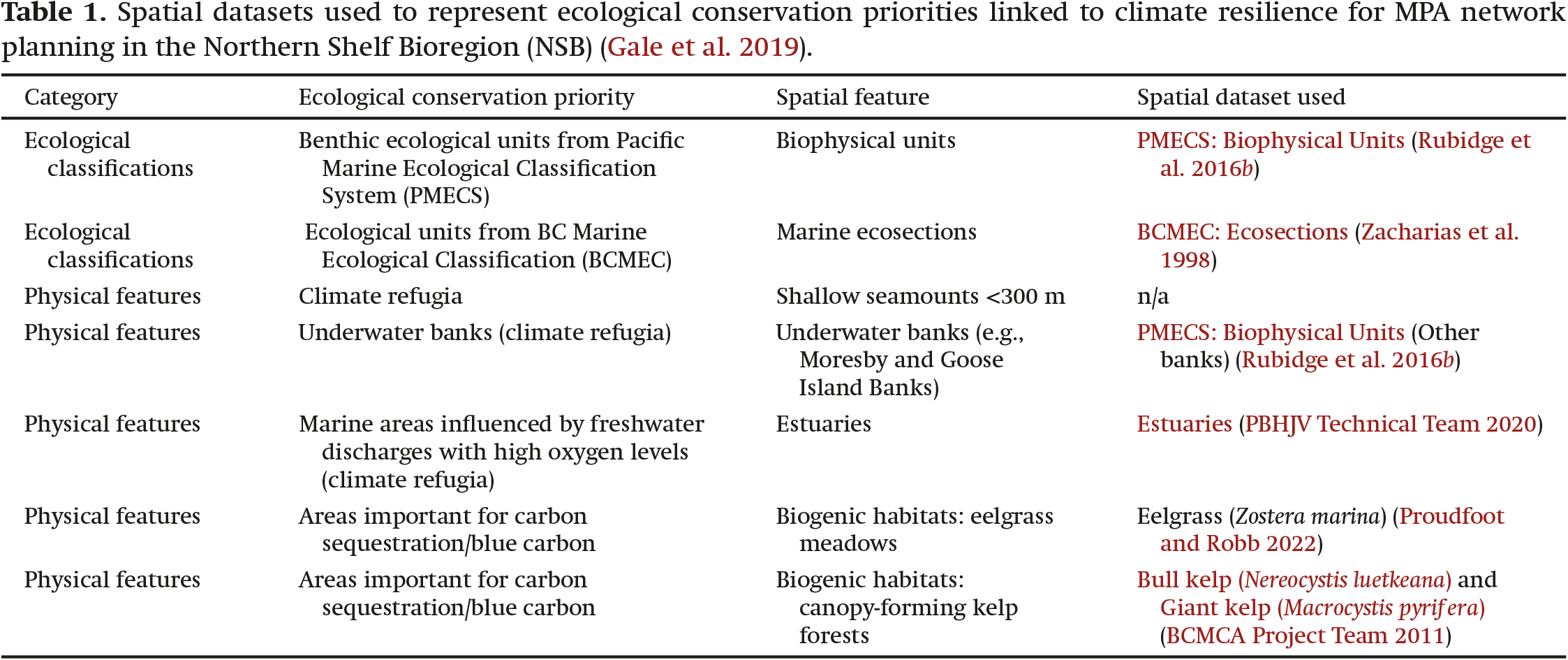
MPAs and MPA networks have more recently been highlighted as tools for climate change mitigation, in part through the protection of “blue carbon ecosystems”, or coastal ecosystems that sequester and store organic carbon, such as eelgrass meadows, salt marshes, and canopy-forming kelp forests (
Jacquemont et al. 2022;
Jankwoska et al. 2022). Often, these ecosystems are targeted for protection in MPAs for their ecological value, where they provide structural habitat for numerous fish and invertebrates, including those of cultural and commercial importance (
Gale et al. 2019;
Rubidge et al. 2020;
Unsworth et al. 2022). However, the protection of these habitats also provides the co-benefit of carbon storage and sequestration, though the amount of carbon fixed and stored may vary regionally (
Krause-Jensen and Duarte 2016;
Röhr et al. 2018). Despite this variation and associated uncertainty around specific carbon fixation rates, there is building evidence on how to more directly include blue carbon ecosystems in MPA design to maximize climate-change mitigation benefits (
Sala et al. 2021;
Epstein and Roberts 2022;
Jankowska et al. 2022).
Recent papers have been calling for more climate-robust or “climate-smart” ocean planning, where MPA networks are a cornerstone of approaches and explicitly managing and designing MPAs with climate change as an overarching factor is key (
Wilson et al. 2020;
O'Regan et al. 2021;
Bryndum-Buchholz et al. 2022;
Buenafe et al. 2023). Adaptation strategies that can be implemented by MPA networks to increase resilience include protecting climate refugia, protecting future habitats of priority species, increasing connectivity, increasing heterogeneity, incorporating blue-carbon ecosystems, and reducing other stressors (
Wilson et al. 2020;
Bryndum-Buchholz et al. 2022). Here, we present a case study on a proposed MPA network in Pacific Canada to illustrate how climate change considerations were built into the network design. Canada has committed to establishing MPA networks as part of its effort to meet international and national targets to protect 30% of their ocean and coastal waters by 2030 (
PMO 2021;
CBD 2022). The MPA network in the Northern Shelf Bioregion (NSB;
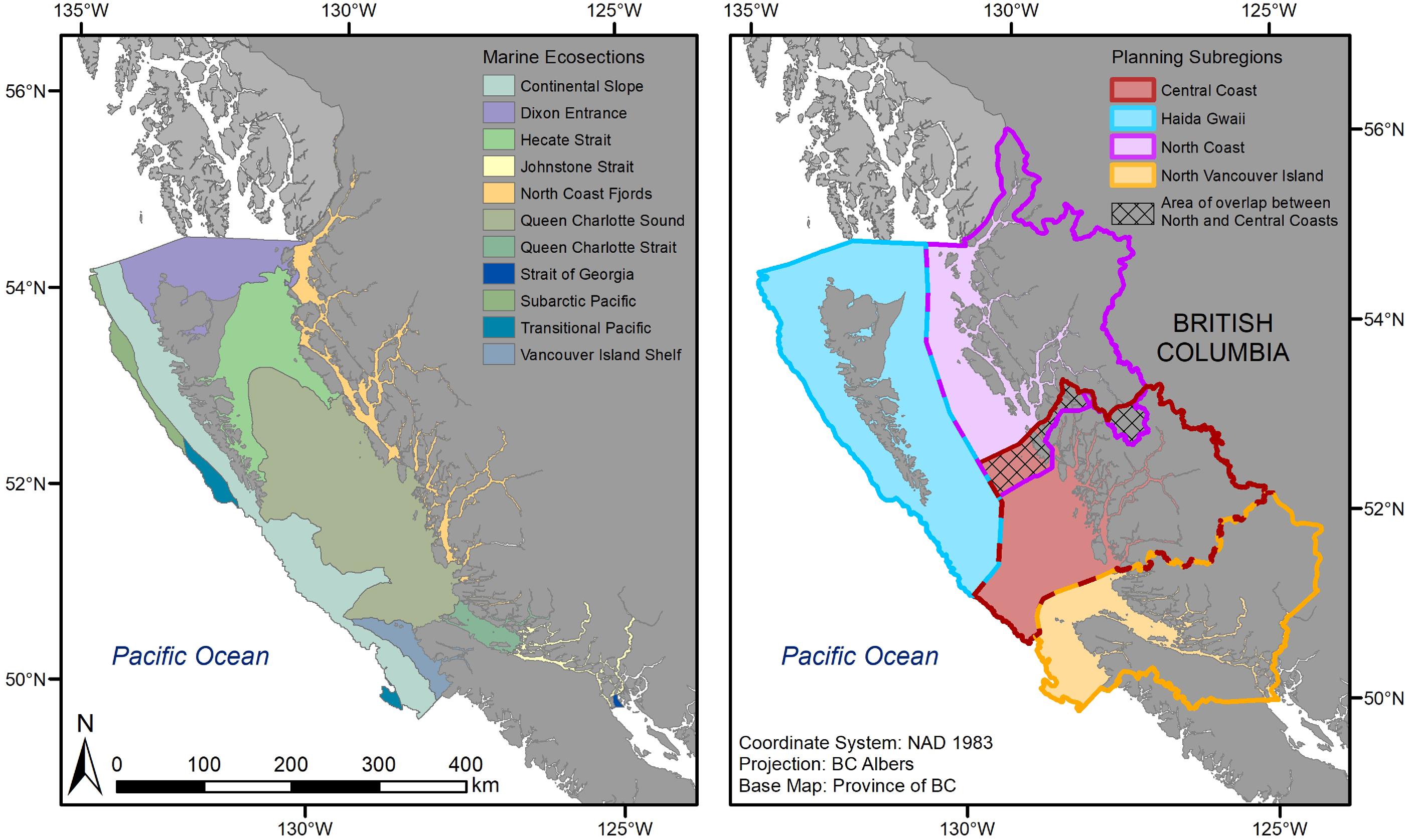
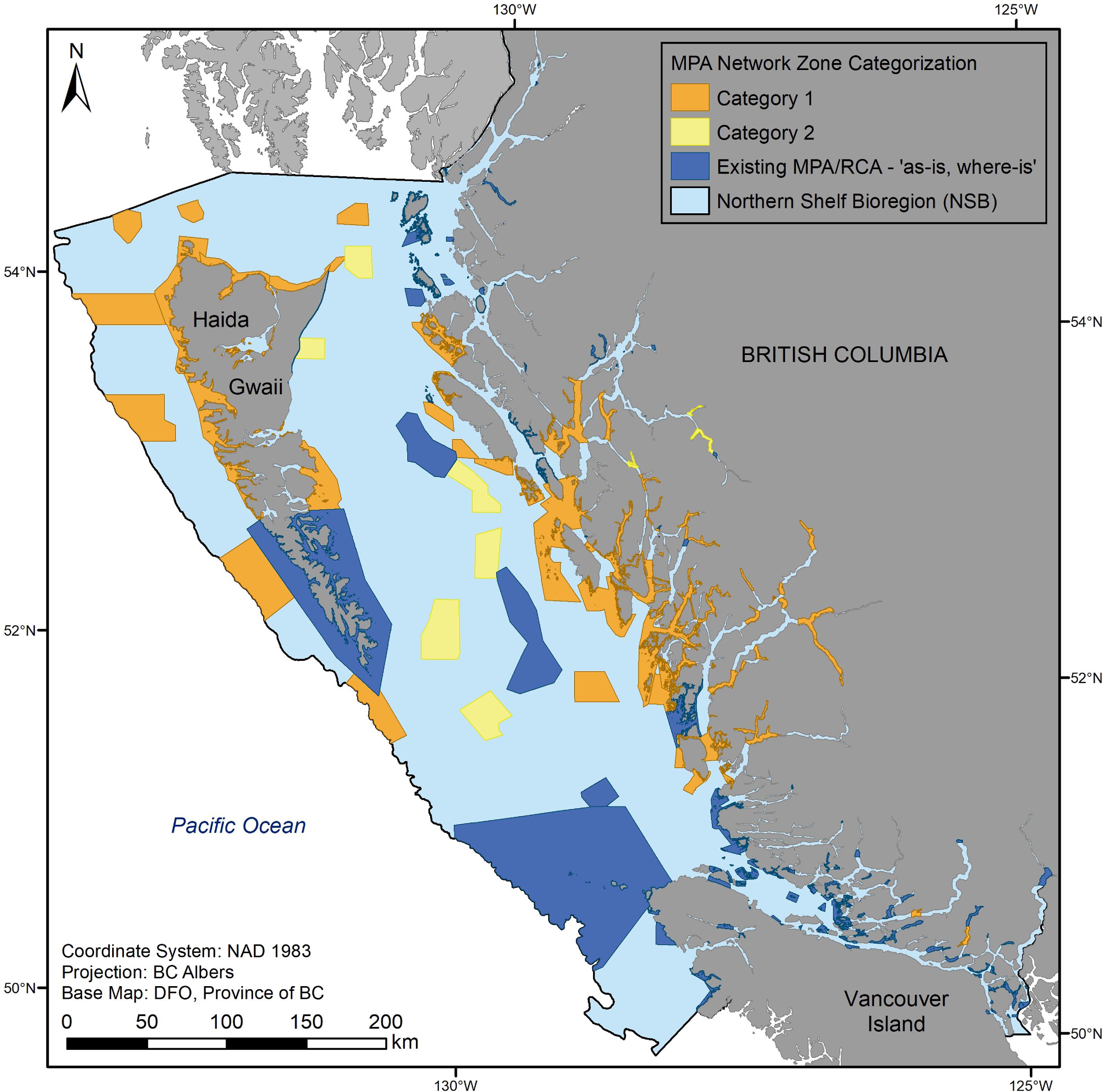
Fig. 1) was announced by the planning governments of 15 First Nations,
1 British Columbia (BC), and Canada in February 2023 (
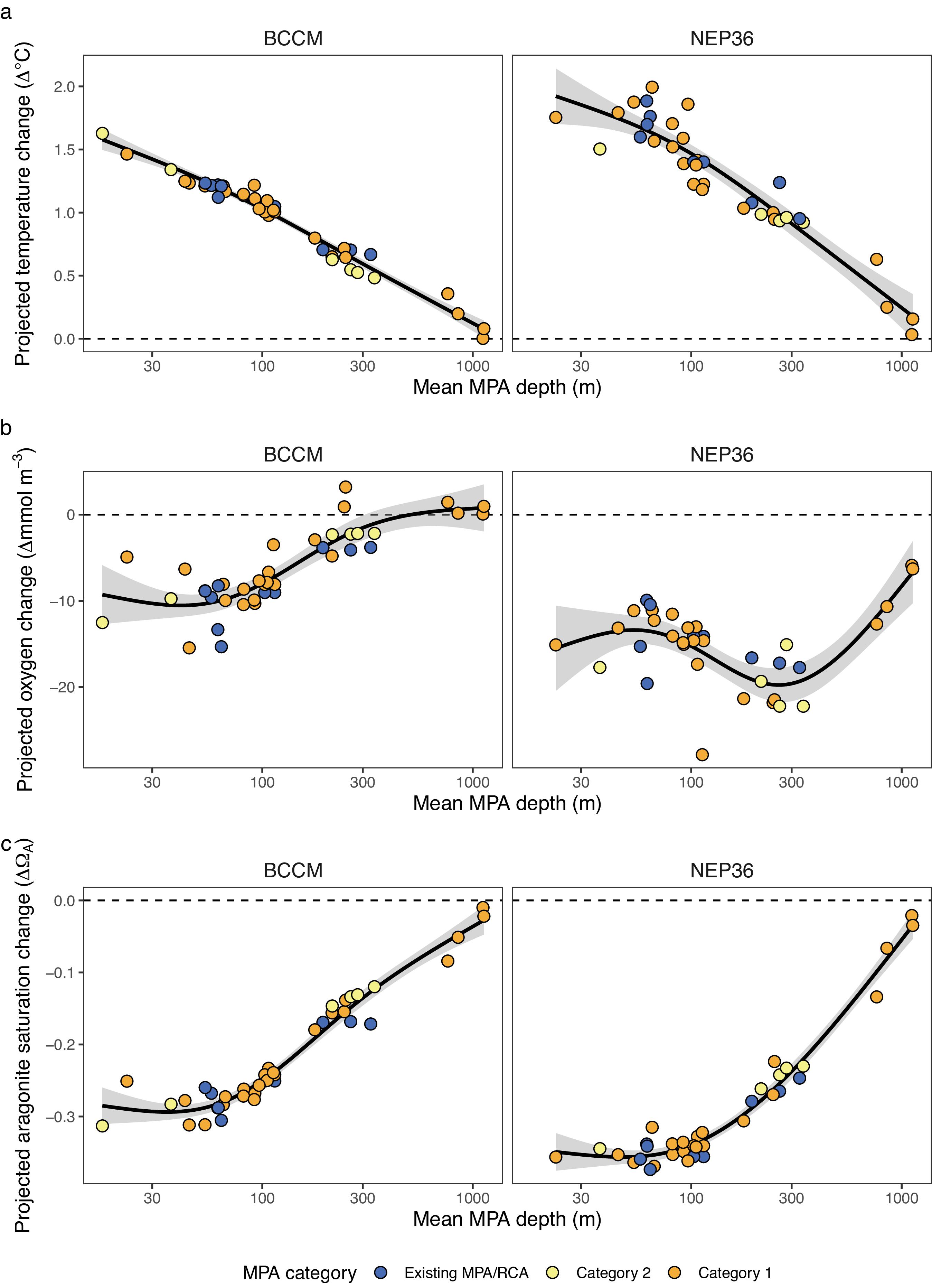
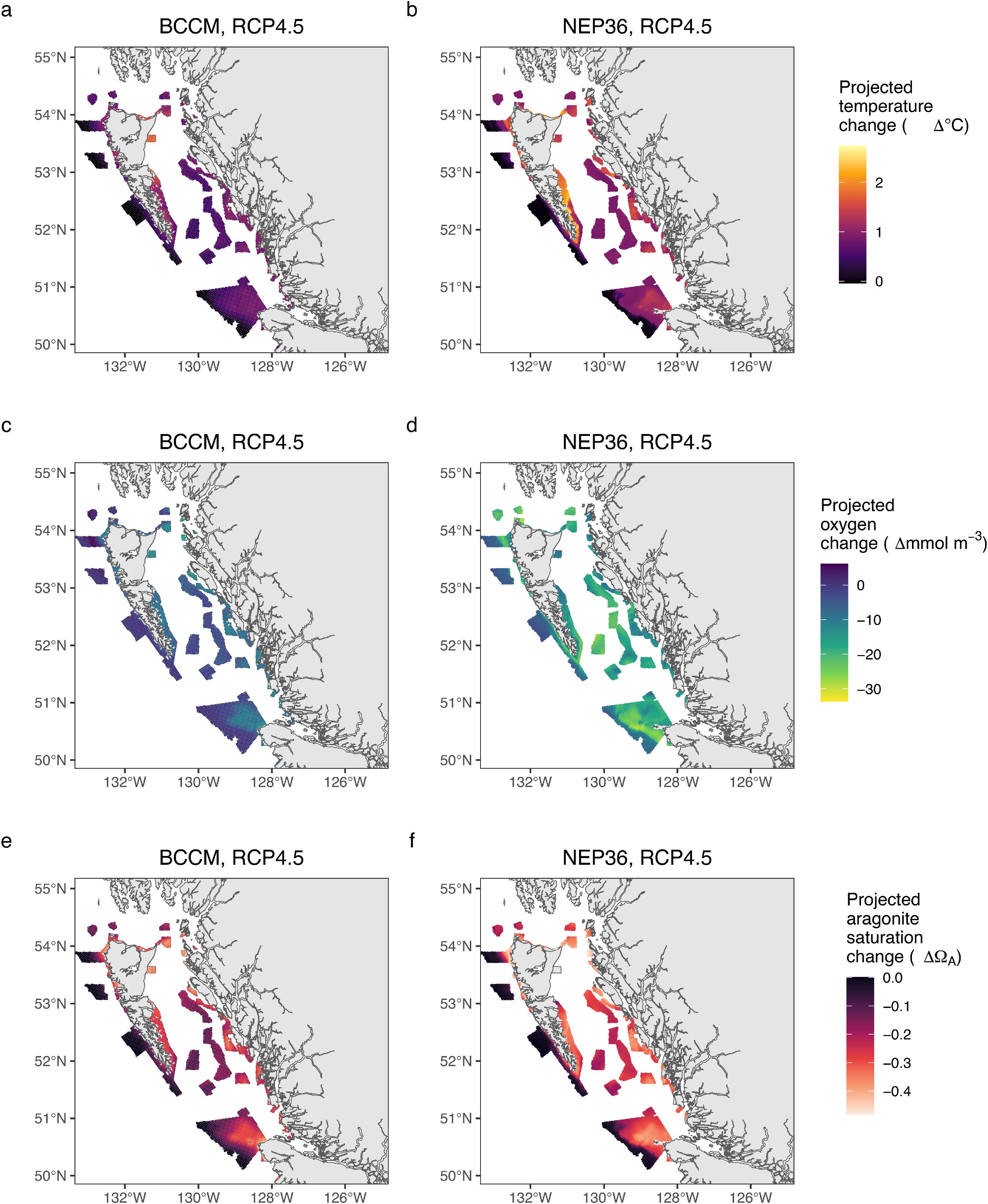
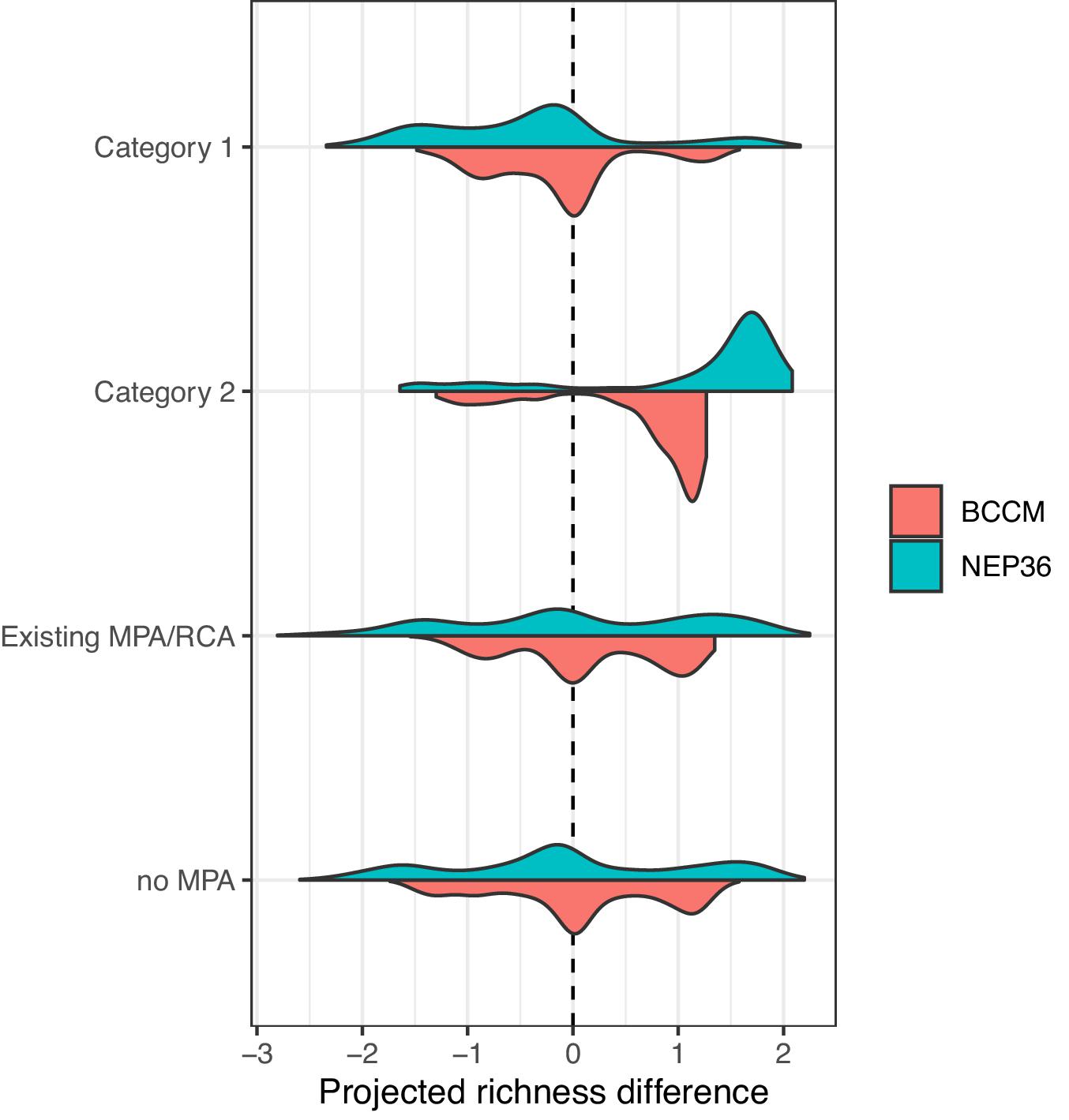
MPA Network BC Northern Shelf Initiative 2023). The objectives of this paper are to (1) evaluate the final proposed network against design strategies and guidelines linked to climate resilience developed and applied by the NSB MPA network technical team (MPATT); (2) assess how the proposed MPA network is projected to change in physical and biogeochemical characteristics (temperature, dissolved O
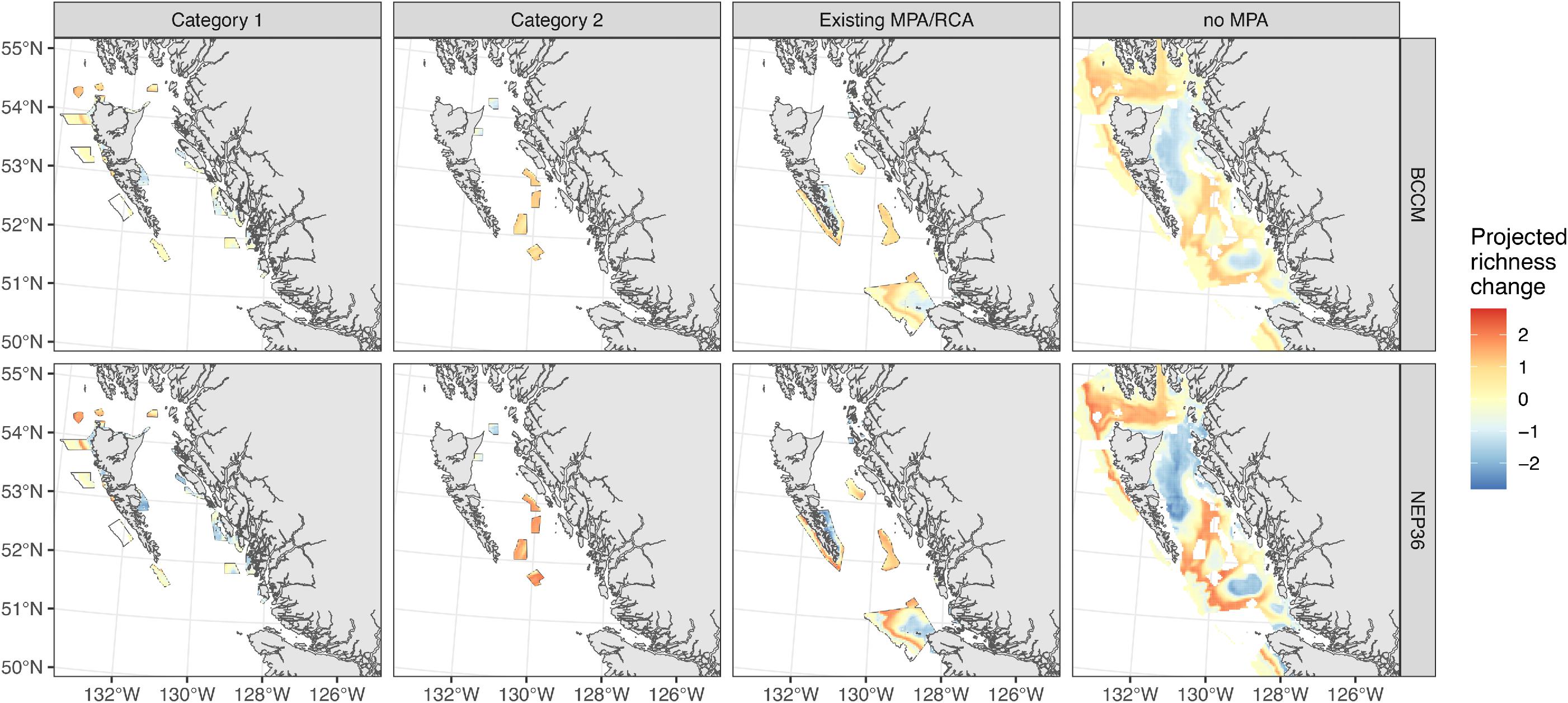
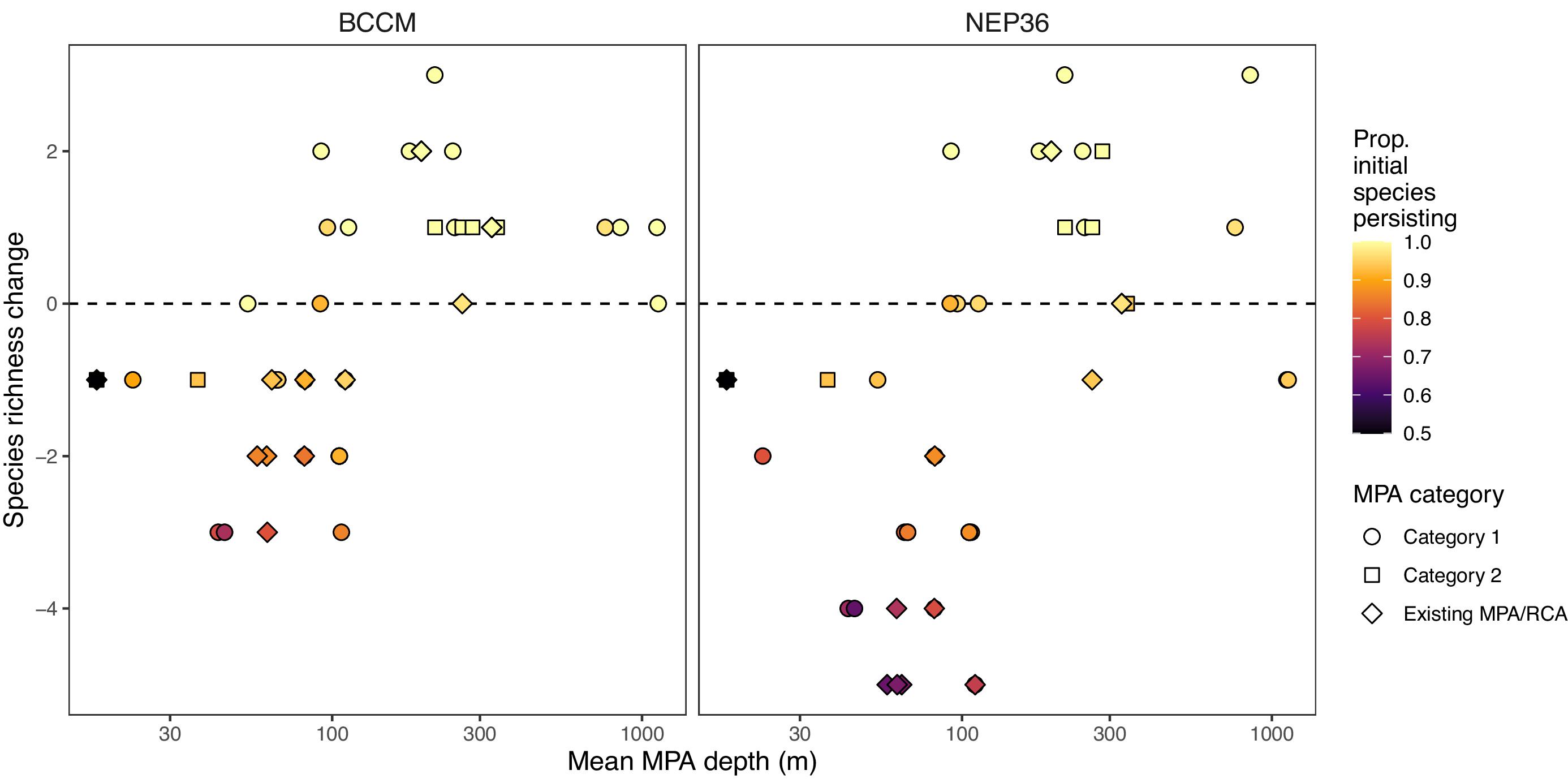
2, and ocean acidification) under potential future climate change; and (3) evaluate how the proposed NSB network scenario will protect the future habitat of demersal fish.
Acknowledgements
We would like to thank S. Diggon, K. Worsley, K. Leslie, J. Dunham, A. Heidt, and all the members of the Marine Protected Area Network Technical Committee for their leadership and participation in the NSB MPA network planning process and for their contributions that helped shape the research in this paper. This includes representatives from the following partners: Gitga'at, Gitxaala, Haisla, Kitselas, Kitsumkalum, Metlakatla, Heiltsuk, Kitasoo/Xai'xais, Nuxalk, Wuikinuxv, Mamalilikulla, Kwiakah, Tlowitsis, and Wei Wai Kum First Nations; Council of the Haida Nation; the Province of British Columbia; and the Government of Canada. This research was funded, in part, by the Fisheries and Oceans Marine Conservation Targets Program.