Introduction
Wild birds are natural reservoirs of avian influenza virus (AIV), but until 2005 they appeared to be mostly refractory to serious disease caused by infection with highly pathogenic AIV (HPAIV;
Liu et al. 2005). However, since 2022, frequent outbreaks, high mortality, and intercontinental spread of HPAIV subtype H5N1 lineage A/goose/Guangdong/1/1996 (Gs/GD) clade 2.3.4.4b have made wild birds a focal point for HPAIV wildlife monitoring worldwide (
Ramey et al. 2022;
Harvey et al. 2023).
Increased detection of HPAIV-attributable morbidity and mortality in poultry, wild birds, free-living mesocarnivores, and marine mammals in recent years has sparked global concern (
Verhagen et al. 2021;
Alkie et al. 2022,
2023;
Banyard et al. 2022;
Caliendo et al. 2022). In Eurasia, HPAIVs are considered to be enzootic, circulating endemically in wild birds (
Verhagen et al. 2021;
Ramey et al. 2022). The recent changes in HPAIV dynamics in wildlife are due to the emergence of HPAIV H5 clade 2.3.4.4b, which appeared in 2014, and now dominates HPAI disease burden globally with sustained transmission in wild birds (
Verhagen et al. 2021). Mass mortality events attributed to 2.3.4.4b have been reported in great skuas (
Stercorarius skua) in Scotland (
Banyard et al. 2022), South American sea lions (
Otaria flavescens) in Peru (
Gamarra-Toledo et al. 2023), and harbour seals (
Phoca vitulina) in North America, among many species (
NOAA 2022). Notably, in 2022, seabirds (e.g., auks, sulids, larids) were particularly affected by 2.3.4.4b, which led to unusual mass mortality events in both North America and Europe (
USGS 2022;
WHISPers, 2023). Despite global efforts to track and understand the H5 2.3.4.4b clade, it is unclear how common HPAIV infection is among migratory seabirds (
Lang et al. 2016).
Murres are a genus of seabirds—also known as guillemots in Europe, turrs in Newfoundland and Labrador, Canada, and as akpa (
⊲b∠) in Nunavut, Canada—that includes common murre (COMU;
Uria aalge) and thick-billed murre (TBMU;
Uria lomvia). They are abundant, migrate across ocean basins (
Frederiksen et al. 2016), and congregate at high-density breeding sites, with potential for high levels of fecal-oral and respiratory transmission of viruses at their colonies (
Huang et al. 2014). Murres have been identified as reservoir hosts of low pathogenic AIV (LPAIV) throughout their range in the northern hemisphere (
Granter et al. 2010;
Huang et al. 2014;
Wille et al. 2014;
Lang et al. 2016), and have now also been found to be infected by HPAIV in the Netherlands (
Caliendo et al. 2022) and Scotland (
Falchieri et al. 2022). In Canada, AIV data from murres are available from 2007–2022, before and since 2021 HPAIV incursion(s) in wild birds of North America.
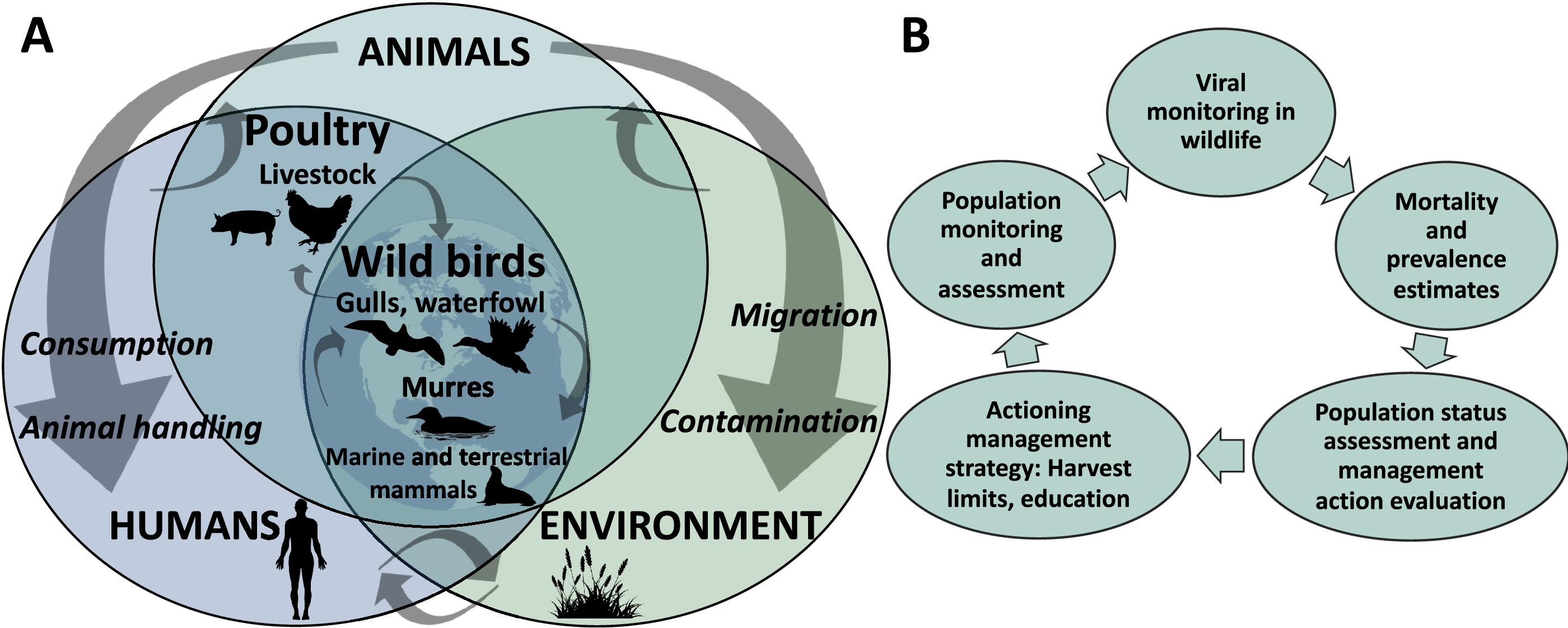
Most wildlife monitoring programs in Canada in 2022 were risk-based, with planning based on known information on AIV in some species. However, this study explores the use of AIV monitoring data at a species-level and how its findings can inform hunters on harvest limits and provide them with guidance regarding risk mitigation and education campaigns. Ideally, disease monitoring is a component of broader population models of mortality and morbidity from multiple sources, which feed into conservation and management priorities and guided actions (
Fig. 1). Ongoing monitoring in wild birds and commercial poultry will help identify deviations from normal disease circulation and burden.
Given this context, this analysis is a case study of monitoring viral prevalence in wildlife within a regional outbreak with elevated urgency in a species with conservation, management, and public health implications (
Stephen et al. 2018). Prevalence of HPAIV and LPAIV infection were calculated in murres sampled across Canada in 2022, and compared to 2007–2021, where monitoring was episodic as part of long-term population monitoring and research priorities. Our objectives were to: (i) report the detection of HPAIV in murres in Canada in 2022 since the HPAIV incursions into North America; (ii) evaluate differences in LPAIV and HPAIV prevalence in murres in 2022 across regions and sampling months; (iii) investigate how AIV prevalence has changed in murres in Canada in 2022 compared to previous years; and (iv) apply power analyses to inform sampling plans for future viral monitoring in relation to the wildlife health policy needs for murres. Further, we discuss the successes and areas for improvement in the monitoring of pathogens in wildlife generally.
Methods
Data sources
This study used data collected across multiple Canadian provinces and territories from the Atlantic, Arctic, and Pacific marine ecosystems. Oral and cloacal swab samples were collected from live/harvested murres in Nunavut (NU) in the eastern Arctic and Newfoundland and Labrador (NL) in the Northwest Atlantic, and from murres found dead due to any cause or found sick in NL, New Brunswick (NB), Nova Scotia (NS), Prince Edward Island (PEI), Quebec (QC) (all Northwest Atlantic), and British Columbia (BC) (eastern North Pacific).
The dataset was compiled from the Canadian Wildlife Health Cooperative (CWHC), Environment and Climate Change Canada (ECCC), and provinces and territories (P/Ts), and included data previously published for samples from NL between 2008 and 2011 (
Granter et al. 2010;
Huang et al. 2014;
Wille et al. 2014), yet-unpublished data from NU from 2011 to present (Provencher et al. in prep.), unpublished historic data, and contemporary 2022 data. Data sources were summarized for 2007–2021 by the number of samples by initial testing laboratories (Table S1), confirmatory diagnostic laboratories (Table S2), and submitting agencies (Table S3), and for the 2022 data, by preliminary testing labs (Table S4) and ECCC project names (Table S5).
Sample collection and laboratory analysis
Although capture and sample collection protocols varied somewhat across sites and personnel, swabs were collected from murres captured live and released, harvested, or found dead or sick. Oral and cloacal swabs from a given individual were often pooled in the same universal transport medium vial. CWHC manages a passive surveillance program for AIV, whereby submitted carcasses are routinely necropsied, swabbed, and tested for AIV. In 2022, due to the unusually high number of mass mortality events, a field-level triage protocol was put in place to limit redundant testing of dead birds, including murres. Therefore, samples represent a subsample of a larger group of found dead murres. Live murres at breeding colonies in NU and NL were often swabbed in conjunction with ongoing research efforts focused on murre populations (e.g., tracking studies, banding programs), as described for the 2022 data in Tables S4–S5. Murres are annually harvested in NU and NL by Indigenous and non-Indigenous hunters, and thus samples from harvested birds were obtained through partnerships with Indigenous governments and organizations in these regions (e.g., Nunatsiavut Government, Nattivak Hunter and Trapper Organization, Sululiit Area Co-Management Committee, and Mittimatalik Hunters and Trappers Organization). Samples from non-Indigenous hunters in NL were obtained opportunistically from individual hunters willing to have their murres sampled, and from murres seized and forfeited to ECCC enforcement officers.
Samples from murres collected in Nunavut in 2007–2021 were primarily sent to the Animal Health Laboratory (AHL) at the University of Guelph, while samples from 2022 were processed by AHL, Memorial University of Newfoundland (MUN), and the National Centre for Foreign Animal Disease (NCFAD). Handling of bird samples at MUN was under protocol 20-05-AL from the Institutional Animal Care Committee and Biosafety Permit S-103. At AHL and MUN, initial screening was conducted by real-time reverse transcriptase polymerase chain reaction (RT-PCR) targeting the AIV matrix (M) gene. Any non-negative samples were further tested by RT-PCR for presence of H5 and H7 subtype viruses. All H5 non-negative samples were sent to the NCFAD for confirmatory testing, isolation, and genomic sequencing. Non-negative includes positive (cycle threshold, Ct < 36) and inconclusive (36 ≤ Ct ≤ 40) results (
CWHC 2022). Thresholds for positive and inconclusive results were in alignment with the standard applied by the NCFAD reference laboratory and used by all laboratories in the Canadian Animal Health Surveillance Network (CAHSN), based on prior literature (
Spackman et al. 2002;
Granter et al. 2010;
Wille et al. 2014). A previous study from which data are included here assigned samples with Ct < 35 as positives (
Wille et al. 2014).
Data curation
Contemporary data for 2022 were downloaded from the CWHC database on 22 November 2022 and joined with additional datasets of hunter-harvested murres from NL provided on 23 December 2022, and murres in BC provided on 27 August 2023. Samples were inspected for duplicate IDs and complete sample dates. Metadata included species (COMU, TBMU), status (live/harvested; found sick or found dead), latitude/longitude (lat/long), province, age class (after hatch year or adult, AHY; hatch year or juvenile, HY), sex (only discernible for murres found dead), capture method, RT-PCR results for M, H5, and H7 genes, lineage assignment to 2.3.4.4b, and origin of genome segments as Eurasian or North American based on whole-genome sequencing (
Giacinti et al. 2023). Samples where RT-PCR M results were missing were removed from the analysis (
n = 1).
Data from 2007 to 2021 were downloaded from the CWHC database on 25 November 2022 and combined with the BC data. Geographic metadata (province, region) were incomplete, therefore lat/long values were used to identify the region of sampling and to compare differences among sampling sites. Two samples had unrealistic lat/long values of “0, −1”. In the first instance, the sampling city was provided and that was used to ascribe a lat/long based on other samples from that city. In the second instance, only province was provided and it was ascribed the lat/long most commonly sampled in the province within two days of the sample. Five samples from Prince Rupert, BC had no sample collection date, only year; since their causes of death were gillnet entrapment, their dates were estimated to have occurred during the Nass and Skeena sockeye salmon (Oncorhynchus nerka) runs in mid-July 2010. Duplicate band IDs were excluded if they were collected in the same sampling month and year (n = 12, including: in Nunavut, n = 2 from 2009 and n = 8 from 2011; in Newfoundland, n = 1 from 2009 and n = 1 from 2011). Samples with missing RT-PCR results for M were removed (n = 171).
Statistical analyses
To compare regional patterns, samples from COMU and TBMU were grouped together; however, the analyses were stratified by bird status: live/harvested or found dead (or sick). Data from NL, NB, NS, PEI, and QC were grouped together as the Atlantic region, while data from BC is referred to as the Pacific region, and NU is referred to as the Arctic region.
Viral period (average) prevalence was calculated by status, year, season (breeding summer months, June–August, vs. rest of year), month, region, province/territory, and site. For murres found dead, the terminology “percentage positivity” is utilized instead of “prevalence” because cause of death was not always known and birds found dead were not representative of the wider population. The nomenclature AIV, LPAIV, and HPAIV are used throughout to denote the presence of virus, whereas we did not investigate the presence of disease, denoted by LPAI and HPAI. AIV positivity (% AIV+) was estimated as the number of samples with non-negative M tests divided by the total number of samples tested, per hundred (Fig. S1). HPAIV positivity (% HPAIV+) was estimated as the number of samples with non-negative M and H5 tests divided by the total number of samples tested, per hundred. LPAIV positivity (% LPAIV+) was estimated as the number of samples with non-negative M but negative H5 tests divided by the total number of samples tested, per hundred. Proportions with 95% confidence intervals were calculated using one group two-sided proportion tests.
Statistical comparisons of the AIV, HPAIV, and LPAIV prevalence across time and space were conducted using two-sided χ
2 tests with Yates continuity correction. Fisher’s exact tests were used if any of the assumptions for a χ
2 test were violated, including that >80% of expected frequencies exceed 5 and all expected frequencies exceed 1 (
McHugh 2013). Statistical tests were summarized by
p-value and power to detect a true association of the estimated effect size (Table S6). Binomial generalized linear models with a logit link function were built separately by status to test the association of the response variables, AIV or HPAIV positivity, with the primary predictor, sampling region, considering inclusion of potential confounding variables, season, species, and age category. Likelihood ratio tests were used to test whether confounding variables improved the goodness-of-fit using forward stepwise selection. Final models were interpreted as adjusted odds ratios.
Sample sizes to target for future monitoring years were calculated using two-sided two-proportions with unequal sample size power analyses assuming the baseline comparison to the % AIV+ or % HPAIV+ among live birds (mean and confidence limits) and number sampled in 2022, with a range of expected changes (primary analysis: 25% increase), resultant effect sizes, power values (50%, 80%), and alpha significance values (0.01, 0.05). R packages used in the analysis are listed in the supplementary materials.
Mapping
Cartographic boundary files were obtained for Canadian provinces from the 2016 Census as NAD83 Lambert datum (EPSG:3348) (
Statistics Canada 2019), for the United States as WGS84 (
United States Census Bureau 2019), and for Greenland as WGS84 (
gadm.org 2022). The US and Greenland were reprojected as EPSG:3348 in units of meters. All lat/long values in decimal degrees were truncated to three decimal points for site-specific confidentiality, assigned the WGS84 (EPSG:4326) coordinate reference system, then reprojected to EPSG:3348. R packages used to generate maps are listed in the supplementary materials.
Results
HPAIV and LPAIV detected among Canadian murres in 2022
There were 427 murres tested for AIV in 2022 across Canada, including 305 live/harvested birds and 122 birds found dead (
Table 1). Live/harvested birds consisted mostly of TBMU (
n = 260, 85.2%), which were sampled in the Arctic via research programs (
n = 248, 81.3%) and in NL within the Atlantic region via hunter-based collections (
n = 57, 18.7%). Dead birds were mostly COMU (
n = 81, 66.4%) and were most widely sampled in the Atlantic where they would have been local breeders (
n = 109, 89.3%), with additional sampling in the Pacific (
n = 13, 10.7%). Among birds where age group was recorded, 96.8% (
n = 212) of live murres were after-hatch year (AHY) and 73.3% (
n = 22) of dead murres were AHY (though age data were mostly missing). Sex in murres can only be determined by internal examination or deoxyribonucleic acid, so was not available for live birds; 61.1% (
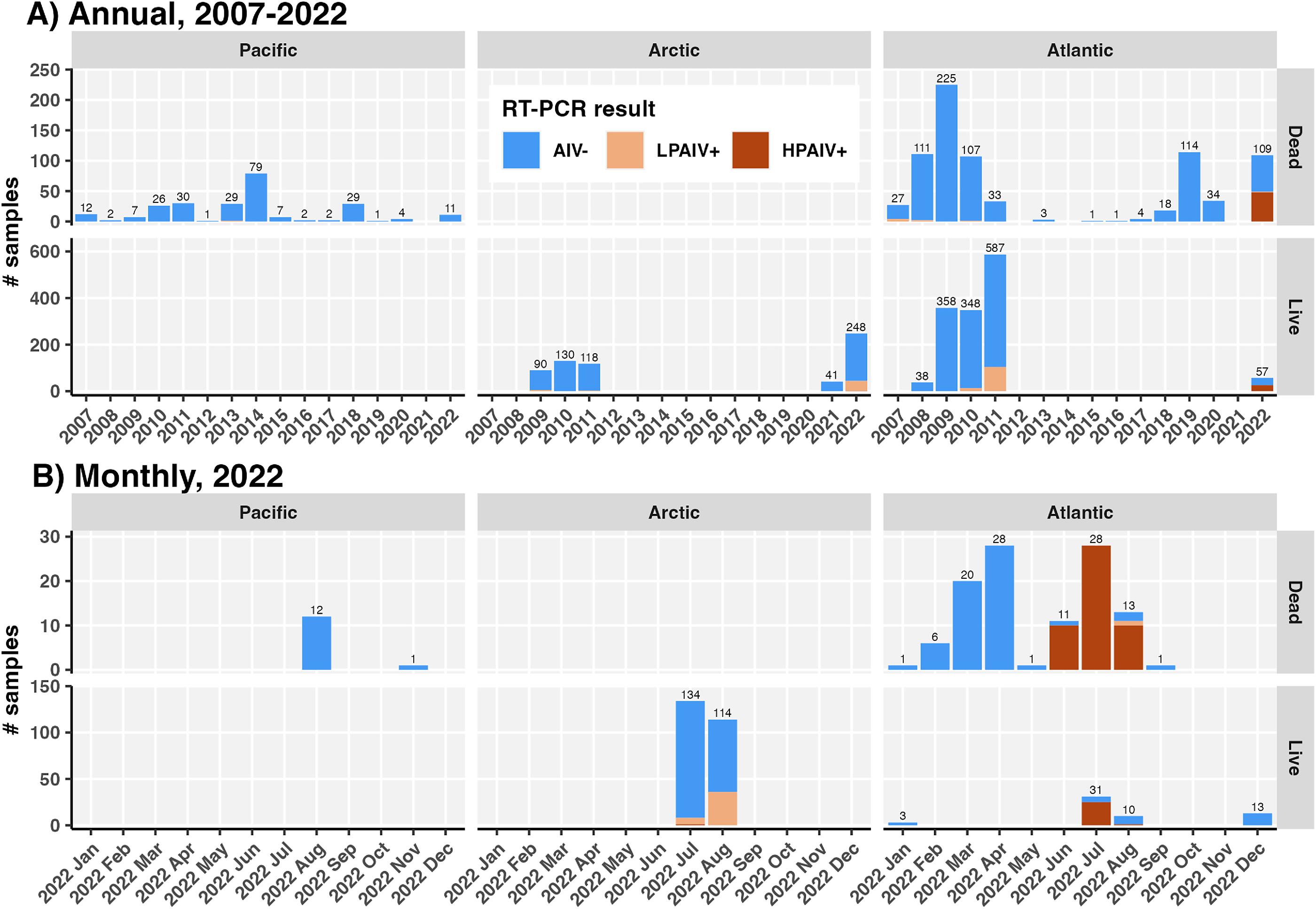
n = 11) of dead birds where sex was determined were male. Live birds were primarily sampled in July (
n = 165, 54.1%) and August 2022 (
n = 124, 40.7%), while samples from dead birds were dispersed through the year (
Fig. 4).
RT-PCR results were summarized as AIV− (M−), LPAIV+ (M+, H5−, or H5 test unavailable), or HPAIV+ (M+, H5+) (Fig. S1;
Fig. 3). There were no detections of H7 in the murres. Our presumption that all the H5+ samples were HPAIV was corroborated by the fact that among the 35 H5+ samples that had their whole genomes sequenced (all from dead COMU), all were within H5N1 clade 2.3.4.4b, of which 33 were inferred to have fully Eurasian lineage genomes, while 2 samples were reassortants with 50% of segments (HA, NA, M, and NS) from Eurasian lineages and 50% of segments (NP, PB1, PB2, and PA) from North American lineages (Fig. S3). The fully Eurasian-origin sequences were sampled between June and August 2022 from PEI, NL, NS, and NB, while the reassortants were identified in PEI and QC within a week of each other in early June 2022. Detailed analysis of these sequences is the focus of a separate study, and beyond the focus of this paper.
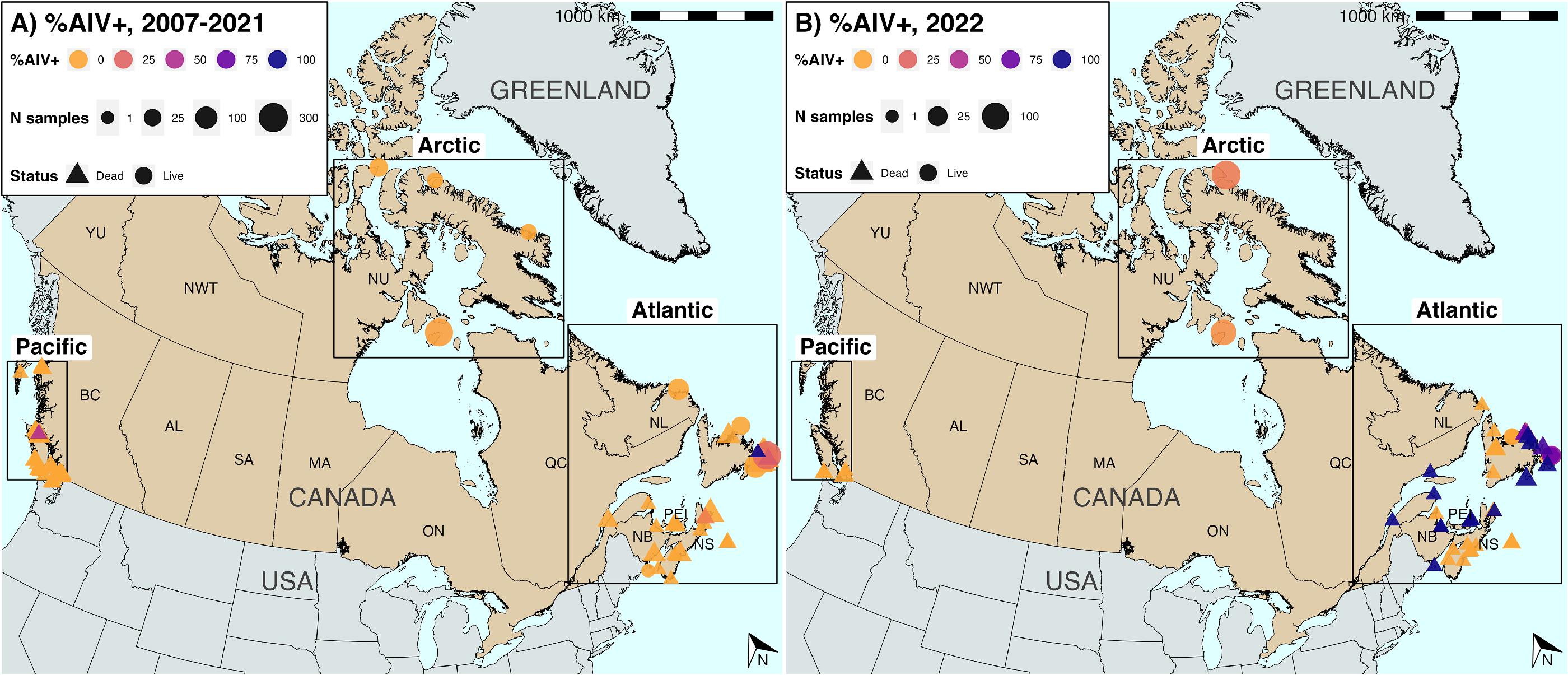
HPAIV was widely detected in murres in 2022 within the Atlantic region: in 48 of 52 dead birds (92.3%) sampled between June and August 2022, and 25 of 31 live breeding birds (80.6%) from July, all most likely locally-breeding COMU (
Figs. 2, and
3). HPAIV was additionally detected in the Arctic in one apparently healthy live breeding bird out of 134 TBMU sampled in June. All the AIV detected in NL was HPAIV. However, LPAIV was detected in 43 of 248 live murres (17.3%) sampled in the Arctic in July and August, and in a single dead bird from NB in August. No LPAIV or HPAIV was detected in murres from the Pacific in 2022 (
n = 13).
Overall, 25.4% (95% CI: 21.4%–30.0%) of all murres sampled in Canada in 2022 were AIV+, of which 59.5% (50.4%–68.1%) were HPAIV+, such that 18.2% (14.6%–22.3%) of all the murres sampled in 2022 were HPAIV+. AIV prevalence was significantly lower in live/harvested murres relative to dead murres, with 23.0% (18.4%–28.2%) AIV+ in live birds compared to 40.2% (31.5%–49.4%) AIV+ in dead birds (χ
2,
p < 0.001; Table S6). Similarly, HPAIV prevalence was significantly lower in live compared to dead birds, with 8.9% (6.0%–12.8%) HPAIV+ in live birds compared to 39.3% (30.7%–48.6%) HPAIV in dead birds (
p < 0.0001). By contrast, LPAIV positivity was significantly lower among dead (0%, 0%–3.8%) than live (16.7%, 12.8%–21.5%) murres (
p < 0.001) in 2022. Comparisons of results from live and dead birds should be interpreted cautiously as the groups represent different proportions of species, regions, and months, with different reporting and detection biases (
Table 1). In NL, live and dead murres did not have significantly different % AIV+, % LPAIV+, or % HPAIV+ (Fisher’s exact test, all
p = 1).
Regional and temporal differences in HPAIV and LPAIV prevalence
There were significant regional variations in % AIV+ (Fisher’s test,
p < 0.0001), % HPAIV+ (
p < 0.0001), and % LPAIV+ (
p < 0.0001) in 2022. Focusing on live birds, AIV prevalence was significantly higher in the Atlantic (45.6%, 32.6%–59.2%) than the Arctic (17.5%, 12.3%–24.3%;
p < 0.0001), as was HPAIV prevalence (Atlantic: 45.6%, 32.6%–59.2%; Arctic (0.6%, 0%–3.7%;
p < 0.0001). However, it should be noted that live bird collection in the Atlantic is a combination of samples obtained from local breeding COMU and harvested overwintering birds, which includes TBMU breeding in the Arctic that migrate south after breeding (
Frederikson et al. 2016). LPAIV prevalence was significantly higher in the Arctic (17%, 11.8%–23.6%) than in the Atlantic (0%, 0%–7.9%;
p = 0.002) in 2022. Although LPAIV was not detected in live birds in the Atlantic in 2022, there was one LPAIV+ sample from a dead COMU in NB. No LPAIV or HPAIV was detected in the Pacific region in 2022 among 13 murres found dead. There were differences in percentage positivity across sampling sites within regions (
Fig. 2; Figs. S7–S9). Pairwise differences in site-level AIV positivity were significantly lower within-regions (median = 14.6) than between-regions (median = 22.2) (Kruskal–Wallis,
p < 0.0001) (Fig. S10).
When the comparison was restricted to live birds sampled in the summer breeding season (June to August), differences between Atlantic and Arctic AIV and HPAIV prevalence were even more pronounced (Table S6) on account of significantly higher AIV and HPAIV prevalence in live birds in the Atlantic in the summer (63.4%, 46.9%–77.4%) compared to the rest of the year (0%, 0%–24.1%; Fisher’s
p < 0.0001). This trend was recapitulated in murres found dead in the Atlantic (summer: 93.9% HPAIV+, 82.1%–98.4%; rest of year: 0%, 0%–7.9%;
p < 0.0001); outside of summer, no HPAIV or LPAIV was detected at any Atlantic sampling sites in 2022 (
Fig. 2). Within the summer breeding season, we compared prevalence in July and August for live birds in the Atlantic and the Arctic separately. AIV prevalence in live birds sampled in the Arctic was significantly higher in August (31.6%, 23.4%–41.0%) compared to July (6%, 2.8%–11.8%,
p < 0.0001); however, this may have reflected differences between breeding colonies sampled in these months. In the Atlantic, HPAIV prevalence was significantly higher in July (80.6%, 61.9%–91.9%) compared to August (10%, 0.5%–45.9%) (Fisher’s,
p < 0.001).
In a multiple binomial model of AIV positivity in live birds in 2022 adjusted for season, murres sampled in the Atlantic had 8.0 (4.0–16.8) times higher adjusted odds of being AIV+ than murres sampled in the Arctic. The odds of HPAIV positivity in live birds in the Atlantic in 2022 were 428 (82–7906) times higher than in the Arctic, adjusted for season. However, confidence intervals were wide and this relationship should be re-evaluated with longitudinal data.
Long term trends in HPAIV and LPAIV prevalence in murres sampled in Canada
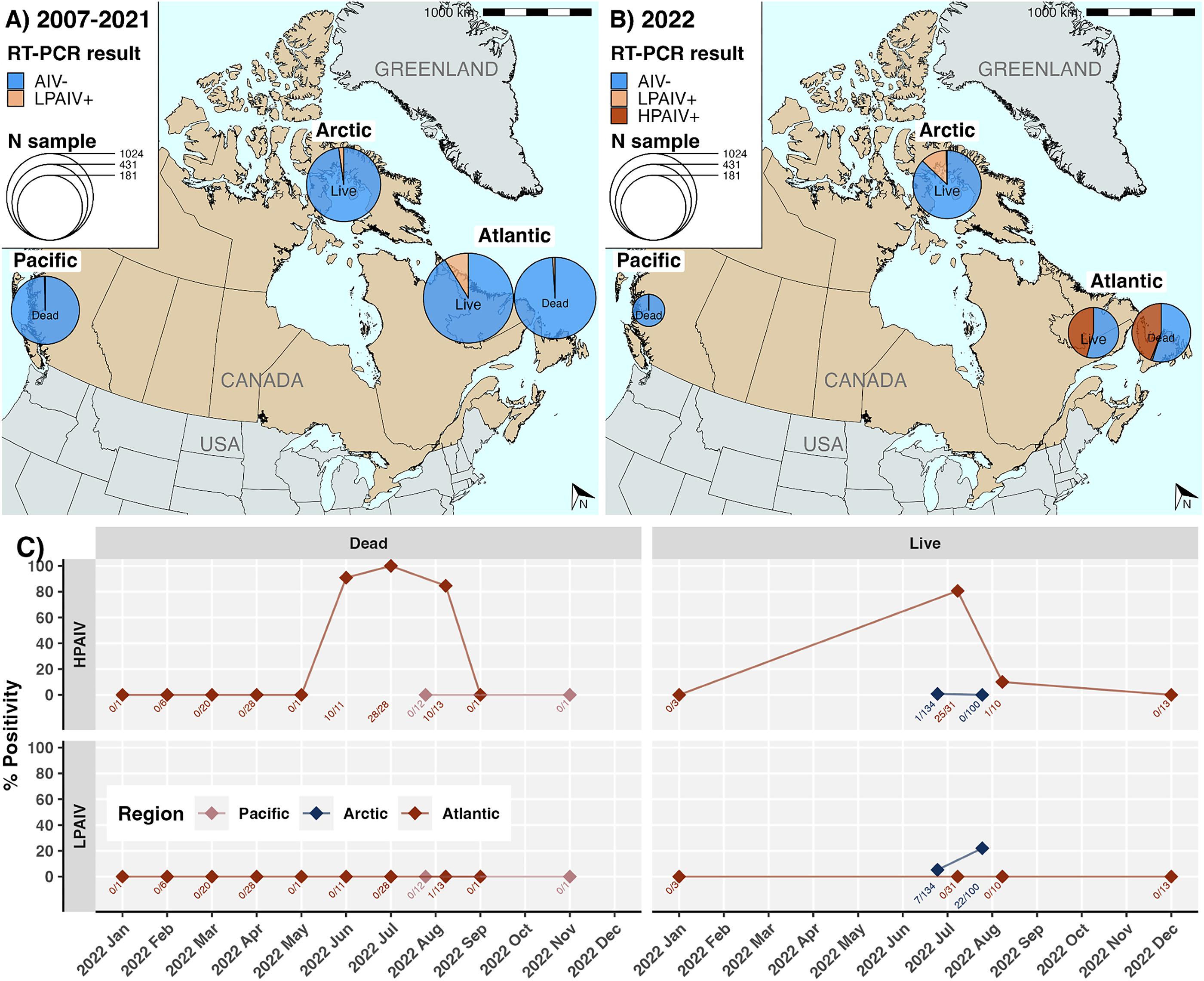
We compared the murre AIV prevalence in 2022 to historical data from 2007–2021, a 15-year period during which 1710 live and 909 dead birds were sampled and tested for AIV, with sampling concentrated primarily pre-2015 (
Table 2). Across 2007–2021, 5.1% (4.3%–6%) of 1710 live birds were LPAIV+ compared to 0% (0%–3.7%) of 909 dead birds, although dead birds included Pacific and not Arctic birds, and live did not include any from the Pacific. H5 was not detected in any of 8 AIV+ samples from dead birds and 125 AIV+ from live birds from August 2007 to October 2013. From October 2013–October 2020, none of 325 found dead birds were AIV+. No dead birds were sampled in 2021. Live birds were not sampled for 10 years until July 2021, when none of the 41 live birds sampled in the Arctic were AIV+.
Across regions and seasons, LPAIV prevalence mostly remained below 5%, except for murres found dead in the Atlantic in summer 2007 (n = 20, 20%, 6.6%–44.3%), as well as live murres in June 2009 in the Arctic (n = 62, 8.1%, 3%–18.5%) and July–August 2011 in the Atlantic region (n = 369, 28.2%, 23.7%–33.1%) (Figs. S4–S6). In the Pacific, LPAIV has only been detected once, in a murre found dead in October 2013 (n = 29, 3.4% (0.2%–19.6%)).
We cannot exclude the possibility that HPAIV was present but went undetected during this time, particularly in 2021 during which no dead birds were tested and live data were exclusively from the Arctic, however there is an absence of evidence. Three samples were H5 non-negative in 2007–2009, but these were subsequently found to be LPAIV H5 subtypes of North American origin. Relative to 2011, the last year prior to 2022 with live bird data from the Atlantic, overall AIV prevalence in live murres in the Atlantic region increased significantly from 17.7% (14.8%–21.1%) to 45.6% (32.6%–59.2%) in 2022 (p < 0.0001). Within that, HPAIV prevalence in the Atlantic increased from 0% (0%–3.9%) in 2011 to 45.6% (32.6%–59.2%) in 2022 (p < 0.0001). Correspondingly, LPAIV prevalence in the Atlantic decreased from 17.7% (14.8%–21.1%) in 2011 to 0% (0%–7.9%) in 2022. In the Arctic, overall AIV prevalence in 2022 (17.7%, 13.3%–23.2%) had increased significantly since 2021 (0%, 0%–10.7%; p < 0.01) and 2011 (2.5%, 0.7%–7.8%; p < 0.001), as did LPAIV prevalence (p < 0.01; p < 0.0001, respectively). Although HPAIV prevalence in the Arctic in 2022 (0.4%, 0%–2.6%) was slightly higher than 2021 (0%, 0%–10.7%) or 2011 (0%, 0%–3.9%), we were underpowered to detect significance. All other reported comparisons were sufficiently powered (Table S6).
Sample sizes for future AIV monitoring
Power analyses were used to estimate sample sizes needed in the future for accurate monitoring of changes over time, assuming moderate changes in disease prevalence relative to 2022 (Table S7). In the primary reported chi-squared analysis, an alpha significance level of 0.05, 25% additive change (a moderate effect size), and 80% power were applied; the effects of these parameters on sample sizes were explored (Fig. S11).
For HPAIV (same as for AIV) in NL, where 2022 prevalence was 45.6% (32.6%–59.2%) in 57 samples, in 2023, N = 63 (42–65) samples would be required to detect this moderate effect size with 80% power and α = 0.05, or 201 (99–220) with α = 0.01 (Table S7). Comparing HPAIV in the summer in NL, prevalence was 63.4% (46.9%–77.4%) in 49 samples, N = 38 (10–74) samples are required in summer 2023 to detect a 25% increase with 80% power and α = 0.05, or N = 92 (16–428) at α = 0.01.
For AIV in the Arctic, where 2022 prevalence (all summer samples) was 17.7% (13.3%–23.2%) across 248 samples, 28 (25–32) samples would be required to detect a 25% increase of moderate effect size, with 80% power and α = 0.05, or N = 45 (39–50) at α = 0.01 (Table S7). To detect a smaller increase in AIV in the Arctic of 15% (Table S8), N = 87 (72–104) samples would be required at α = 0.05 and 80% power. For HPAIV in the Arctic, where 2022 summer prevalence was 0.4% (0%–2.6%), to detect a 25% increase, N = 9 (7–14) samples would be required at α = 0.05, or N = 14 (11–21) at α = 0.01. To detect a 15% increase in HPAIV, N = 18 (13–30) samples would be required at α = 0.05, or N = 28 (20–47) at α = 0.01. Where evidence suggests prevalence differs across sites within regions but is similar across months in the summer breeding season, we conservatively recommend approximately 100 samples be tested seasonally at each site, where resources are available and monitoring systems more well-established, for accurate estimation of small to moderate changes in AIV and HPAIV prevalence in murres with an α between 0.01 and 0.05. Collecting samples at multiple time points within the breeding season from the same sites would increase the probability of detecting rapidly transmitted pathogen outbreaks and provide more accurate estimates of average period prevalence.
Discussion
HPAIV H5 clade 2.3.4.4b viruses were widely detected in Canada in 2022 in live and dead murres sampled in the Atlantic and a single live murre from the southeastern Arctic. AIV and HPAIV prevalence in live birds were significantly higher in the Atlantic than Arctic, and were significantly higher during summer months than the rest of the year. HPAIV has not yet been detected in murres in the Pacific, but sampling has been sparse. In comparison with 2007–2021, a period during which HPAIV was not detected in murres, HPAIV and AIV prevalence were significantly elevated in 2022 in the Atlantic and the Arctic.
HPAIV prevalence was low in the Arctic in 2022, but LPAIV prevalence had increased. It is unclear to what extent the relatively higher frequency of LPAIV versus HPAIV in the Arctic is affected by sampling gaps, geographic isolation from affected flyways (lack of opportunity), or other ecological factors. Sampling efforts for AIV monitoring in murres from 2007 to 2022 were highly variable and not comprehensive across breeding colonies, therefore it is possible that HPAIV circulated below detection levels in 2021 in particular; however, there was also a lack of detected excess morbidity or mortality at breeding colonies in 2021. The absence of HPAIV detections in Arctic murres in 2021 corroborates a phylogeographic study that found northern seabird colonies were not implicated among wild birds importing HPAIV in 2021 from Europe to North America via the Atlantic (
Caliendo et al. 2022). The patterns of AIV geographic diffusion and interspecies transmission must also take into consideration both virus and host diversity, host behavior, summer temperatures, and geographic distance (
Hicks et al. 2022).
We recommend that in future monitoring years 100 samples be collected per site where possible, or per province, to be sufficiently powered to detect moderate increases in HPAIV or AIV prevalence. Additionally, sampling at the same time of year and at the same breeding colonies over time would reduce spatial and temporal (seasonal) bias in sampling, thereby providing more informative data for long term trends assessment. We also recommend recording population parameters, especially annual survival (
Frederiksen et al. 2021), alongside disease monitoring to provide more robust information for potential population-level effects.
All the H5 sequences from murres in 2022 fell within clade 2.3.4.4b, and this clade accounted for nearly all the AIV detected in the Atlantic, which is not unexpected based on its preponderance in Canada and the United States (US) since 2021. H5 clade 2.3.4.4b was first detected in Canada in November 2021 in a great black-backed gull (
Larus marinus) in St. John’s, NL and then on an exhibition farm amid mass mortality in December 2021 (
Caliendo et al. 2022). Phylogenetic analysis of samples from a bald eagle (
Haliaeetus leucocephalus) and broiler chickens in BC from February and April 2022 suggested H5 2.3.4.4b was likely first introduced to North America in 2021 via migratory birds through both the Pacific and North Atlantic flyways (
Alkie et al. 2022). Prior to the 2021 2.3.4.4b sub-epidemic, high pathogenicity H5 had not been detected since 2015, when H5 clade 2.3.4.4 was identified in BC within small flock chickens and a Cooper’s hawk (
Accipiter cooperii;
Berhane et al. 2016). From 2014–2015 in the Pacific region of the US, clade 2.3.4.4 (originated from H5N8 then reassorted into H5N1, H5N2) was detected in 2.4% of hunter-harvested birds (
Bevins et al. 2016). By June 2015, H5 2.3.4.4 had disappeared in poultry due to interventions such as culling and quarantine, and enigmatically, the clade also appeared to have disappeared in waterfowl (
Krauss et al. 2016). However, Ramey et al. responded that this constituted weak evidence of disappearance since the majority of negative samples were collected before the outbreak, in states and provinces where large outbreaks were not reported, and from seabirds, shorebirds, and gulls, as opposed to waterfowl from the order Anseriformes (
Ramey et al. 2016); therefore, it is unclear whether H5 2.3.4.4 circulated below detection levels or outside of well-sampled areas, or was indeed eradicated in North America after 2015. Possible mechanisms for this apparent disappearance include existing immunity, antigenic determinants in wild aquatic bird populations, and the cyclic nature of subtype dominance (
Krauss et al. 2016). It remains to be seen whether accrual of population-level immunity to H5 2.3.4.4b in wild birds will result in a shift to another subtype or clade, or whether something intrinsic to this clade permits long-term establishment in the wild bird reservoir.
Sampling bias and observational gaps limit the generalizability of our findings. The limitations of small sample sizes and the occurrence of rare events have been considered regularly in ecology (
Dixon et al. 2005;
Sequeira et al. 2019). When pathogens circulate in wildlife at low prevalence, sample size can influence case ascertainment. The idea that “if you look hard enough you will detect something” (e.g.,
Mallory et al. 2004) is important to consider when evaluating how prevalence may change over time. While AIV monitoring in murres has not been consistent across the study period, sampling in 2022 did not greatly outpace 2021 in the Atlantic and Arctic; approximately 100 murres in NU were tested annually for AIV prior to 2022. Spurred on by the global concerns around HPAIV, murres were tested in the summer of 2021 as part of an anticipatory effort to increase surveillance (
Fig. 3A). Given that sampling effort has not significantly changed, but we see differences in AIV and HPAIV prevalence, this suggests that these are likely reflecting population-level changes. Other sampling biases that may have affected the results are ease of access for live and dead birds, spatially clustered sampling, survival bias among live birds, and pathogenicity bias in birds found dead.
By aggregating data into regions, this analysis was subject to bias due to the modifiable aerial unit problem, whereby summaries are affected by the size and shape of the aggregation unit (
Dark and Bram, 2007). Pairwise site-level differences in AIV prevalence were significantly lower within- than between-region, suggesting that regional delineations reflected to some extent meaningful ecological, migratory, and geographic groupings. In a related point, we detected differences in AIV prevalence between breeding colonies and further sampling is required over time to understand if this is a reproducible occurrence. This is consistent with the spatiotemporal variation in AIV prevalence that has been described in wild migratory birds of North America (
Munster et al. 2007).
There is an increased need to have up-to-date estimates of prevalence, disease burden, and mortality attributable to HPAIV to inform seabird management and public health risk assessments. At the population level, monitoring viral prevalence and disease burden to determine background and AIV-associated mortality is critical. Disease burden estimates can be incorporated into population status models that consider multiple sources of mortality, where mortality exceeding a threshold can be used as a criterion to warrant taking action with wildlife management tools such as harvest limits and education (
Fig. 1B).
Gaps in viral monitoring make it challenging to detect outbreaks and understand whether they are increasing in frequency or severity. Improving viral monitoring systems for data collection, curation, and analysis in wildlife will improve our ability to rapidly detect, respond to, and learn from outbreaks (
Verhagen et al. 2021;
USGS 2022;
WHISPers 2023). There is a critical need to develop evidence-based, wildlife viral monitoring tools by collaborating across agencies to analyze existing data, design programs that are cost-effective, and produce high quality data (
Stephen et al. 2004;
Giacinti et al. 2022). In Canada, some of the challenges faced by wildlife health surveillance programs include a lack of a regulatory framework surrounding cooperation and coordination across government levels and agencies, as well as complex sampling biases and competing priorities (
Giacinti et al. 2022). This results in waves of interest, and the monitoring taking place is resource-dependent and often triggered by some event, such as mass mortalities in wild birds or domestic culling, accompanied by economic losses and public impetus. Resources wane with weakening public interest and eventually are diverted to the next emerging issue. This can be seen in the patterns of AIV monitoring in murres in Canada as described above.
From a murre management and conservation perspective, combining viral monitoring with other data, such as presence of other microbes, contaminants, and immune function (
Mallory et al. 2010), that affect birds’ survival can reveal cumulative and pleiotropic effects on acute survival and reproduction. In birds, for example, higher prevalence of parasites has been linked to reduced reproductive fecundity (
Hudson and Dobson, 1991), and high contaminant levels of mercury may be correlated with more frequently severe viral infections (
Teitelbaum et al. 2022). Human activities resulting in environmental and climate changes, as well as habitat loss could alter viral persistence in the environment and provide additional opportunities for viral reassortment and cross-species transmission (
Morin et al. 2018;
Carlson et al. 2022). Pathogen monitoring is only one aspect of a more comprehensive understanding of wild birds’ population dynamics.
Murre populations experience mortality for a variety of reasons outside of HPAIV, including as bycatch in fisheries gear, oil spill events, poor nutrition, extreme weather, and harvest (
Gaston et al. 2002;
Wiese et al. 2004;
Wiese and Robertson 2004;
Clairbaux et al. 2019;
Frederiksen et al. 2019). Although tracking banded individuals and birds with logging devices has clarified murre wintering strategies and site fidelity (
McFarlane Tranquilla et al. 2014), as well as size and composition of wintering populations (
Frederiksen et al. 2016), tracking survival of birds across their range could add a finer resolution of when, where, and possibly how birds are dying. Serological samples paired with swabs from live birds would clarify the role of prior exposure and immunity towards estimated incidence and survival rates. Continued monitoring for HPAIV in birds found dead, including beached bird surveillance, is an additional important surveillance tool to quantify changes in HPAIV diversity and associated mortality.
The population-level impacts of HPAIV are relevant for the sustainability and availability of murres as a food source for subsistence and sociocultural value. The results presented here suggest that during this harvest season, typically 3.5 months between September to March in Atlantic Canada, murres experience low to negligible levels of active infections. The hunter health risk of AIV is low if harvested birds are handled and cooked properly (
Canadian Food Inspection Agency 2022). Nonetheless, this information is important to be able to share with harvesters as it relates to bird handling and food security questions. Thus, even though AIV prevalence during the winter murre harvest is likely low, continued winter screening of murres to inform public health advice, and detect any changes in winter prevalence of AIV is advisable, again at the same sample size targets (∼100 individuals per region).
With continuous measurements and predefined action plans, HPAIV monitoring could evolve into HPAIV surveillance (
Hoinville et al. 2013). There are often no predefined conservation and management policies or actions for surveillance, but they can include adjusting hunter permitting and safety guidance, education campaigns to reduce exposure, or wildlife vaccination for targeted species of conservation concern, based upon estimates of disease prevalence to counterbalance excess bird mortality (
Fig. 1). For the case of murres in Canada, surveillance of AIV is important for both tracking the virus and pathology in murre populations as part of a conservation program, and to inform public health discussions around hunter safety and guidelines related to human ingestion. Within a One Health monitoring framework, which emphasizes the connections between the health of humans, wild and domestic animals, and environments, interdisciplinary collaboration is key to understand, control and prevent zoonoses, and more broadly to enhance ecosystem health (
Gibbs 2014).