Introduction
Indigenous and local people have detailed knowledge about the biodiversity of animals and plants (
Berkes et al. 2000;
Huntington 2011). This knowledge has received increased attention from international forums and policy-oriented research due to its relevance to biodiversity conservation (
Hill et al. 2020;
Ogar et al. 2020). The Indigenous and local knowledge (ILK) system includes multiple domains, and one of these domains refers to the recognition, naming, and classification of organisms (
Berlin 2014;
da Silva Mourão and Barbosa Filho 2018). ILK related to recognition of biodiversity, which has been extensively studied in the fields of ethnobiology and ethnotaxonomy, is the focus of the present study. These areas of study, focused on interactions of people with the environment, can help advance understanding of how knowledge is transmitted and the associated mental models therein (
Medin et al. 1999). The naming and knowledge of animals and plants may be influenced by their respective utility to people or by intrinsic characteristics that make organisms more noticeable (salience), such as abundance, coloration, or size among others (
Begossi and De Figueiredo 1995;
Hunn 1999;
Ramires et al. 2012;
Berlin 2014).
Hunn (1999) observed four factors determining cultural recognition and natural discontinuities: phenotypic salience, ecological salience, size, and cultural salience. Notwithstanding the well-developed research on ethnobiology of medicinal plants (
Hanazaki et al. 2000;
Begossi et al. 2002;
Vandebroek et al. 2008;
Vandebroek and Balick 2012;
de Andrade et al. 2021), studies on ethnobiology have addressed several kinds of animals, such as birds (
Diamond and Bishop 1999), mammals, birds and snakes (
Atran 1999;
Fita et al. 2010;
Prado et al. 2014), birds and fish (
Hunn 1999), cetaceans (
Souza and Begossi 2007), insects (
Lima et al. 2016), and bats (
Rego et al. 2015).
The Amazon basin has the world’s richest fish diversity (
Dagosta and De Pinna 2019), which is influenced by the seasonality of water levels (flood pulse) and a diversity of aquatic habitats such as the river channel, tributaries, lakes, and floodplain forests (
Junk et al. 1989;
Sioli 2012). This high diversity of fish and habitats is exploited by widespread small-scale freshwater fisheries (
Hallwass et al. 2013b;
Hallwass and Silvano 2016), which sustain one of the highest per capita consumption of freshwater fish in the world (
Isaac et al. 2015;
Begossi et al. 2019;
Ferreira et al. 2022). However, many fish species used as food are data deficient in that they lack biological information or conservation assessment in the Brazilian Amazon (
Begossi et al. 2019). Previous research has corroborated fishers’ knowledge on fish ecology in the Brazilian Amazon (
Silvano et al. 2008;
Batista and Lima 2010;
Hallwass et al. 2013a;
Silvano and Begossi 2016;
Nunes et al. 2019;
Hallwass et al. 2020b;
de Souza Junior et al. 2020). Nevertheless, few studies have specifically addressed the recognition and naming of the rich fish diversity by fishers in the Amazonian rivers (
Begossi and Garavello 1990;
Begossi et al. 2008), especially with respect to a diverse set of fish species. According to
Atran (1993), any criteria, whether utilitarian or symbolic, should be culture-specific. Therefore, Amazonian small-scale riverine fishers are part of the same culture, which allows comparisons.
Our study has the goals of, first, checking the extent of riverine fishers’ knowledge (recognition and naming) of 115 and 119 fish species (65 species shared between the two rivers), respectively, in the Negro and Tapajos Rivers, two rivers with high fish diversity in the Brazilian Amazon. Second, we elaborated an index to quantify fishers’ knowledge and to investigate its relationship with importance to fisheries, fish abundance, and fish size of each fish species. Our overall hypothesis is that fishers’ knowledge would be positively related to fisheries importance, abundance, and size, as bigger fish would be better known (
Begossi and De Figueiredo 1995;
Begossi et al. 2008). Third, we compared the fishers’ perceptions on importance of fisheries and the abundance of each fish species with independent data from fisheries and fish sampling, respectively. Fourth, we analyzed fishers’ knowledge on aquatic habitats where each fish species can be found.
Data analysis
We first summarized the information on the number of species that were known and named by most fishers in each river. We then calculated a simple knowledge index (hereafter, KI) for each species in each river, following the formula:
where KI is the knowledge index;
N names is the number of fishers who provided the same name to the fish,
N do not know is the number of fishers who did not know the fish, and
N total of interviews is the total number of interviewed fishers. This index ranged from −1 (no fishers knew the fish) to + 1 (all fishers recognized the fish through a unique name). This fish name could be either a single word (e.g., pescada) or two combined words (e.g., tucunaré pinima), which are usually referred to, respectively, as generic and binomial names in studies of ethnotaxonomy (
Begossi et al. 2008). However, diminutives where considered to be the same name (e.g., mandizinho was considered the same as mandi). We considered that the number of names of each species indicated lower consensus among interviewed fishers regarding the species name, whereas receiving the same name by most or all fishers indicated the distinctiveness of each species. The KI was negatively related to the number of names provided for each species in the Negro (nonparametric Spearman (rs) = −0.19,
p = 0.04,
n = 115) and Tapajos (rs = −0.8,
p < 0.0001,
n = 119), besides being negatively related to the number of species that received the same name in the Negro (rs = −0.22,
p = 0.02,
n = 115) and Tapajos (rs = −0.26,
p = 0.004,
n = 119). We thus considered that the proposed KI is a reliable quantitative indicator of fishers’ knowledge about each fish species.
We also calculated a fishing index (FI) and abundance index (AI) to quantify fishers’ perceptions respectively on fish species importance to fishing and abundance, by assigning numerical values to the closed answers provided by fishers regarding questions 3 and 4: none (0), few (1), average (2), many (3), which were averaged among all answers. These indexes varied from 0 (a fish species that was not fished or did not occur in the region) to 3 (a fish species that all fishers often catch or considered to be abundant). We arbitrarily assigned 0 values for both FI and AI for the unique fish species not recognized by any of the interviewed fishers in the Negro River.
We compared the KI with both FI and AI through rs correlation analyses, as the data were non-normal, even after log-transformation. We compared the average number of names cited for each species, between species recognized by all fishers, and species not recognized by at least one fisher, through Mann–Whitney nonparametric U test, in both rivers.
We organized a matrix for each river with fish species and general habitat categories (seven in Negro and five in Tapajos) mentioned by fishers, considering the total number of citations by fishers for each species in each habitat. We then made a multivariate principal component analysis (PCA) using the Euclidean similarity index, to group fish species according to the most cited habitats.
We also correlated the FI and AI with data from our previous studies conducted in 2016 and 2017, respectively, on biomass caught and frequency (number of fish landings on which the species was caught) of each species on fisheries (
Hallwass et al. 2020a) and the biomass of each species collected through fish samples (
Silvano 2020) in the same studied fishing communities in both rivers. This database consisted of 3,830 fish landings (fishing trips) recorded by the fishers (
Hallwass et al. 2020a,
Keppeler et al. 2020) and 13,624 individuals of fish sampled by using gillnets in 16 sampling sites (
Silvano 2020). We assessed the abundance in biological samples of the same species shown in the photos, whereas the biomass and frequency of each species on fish landings were assessed by comparing the common names cited by fishers (
Supplementary Tables S1 and
S2) with the common names recorded by fishers during the participatory monitoring of fish landings (
Hallwass et al. 2020a). However, the fish names recorded in fisheries were often more general, thus corresponding to more than one, sometimes to several species (e.g., the name piranha corresponds to several species of the genus
Serrasalmus). In such cases, we included the same values of biomass caught and frequency in fish landings for all species that received the same name. Some fish landings showed either more detailed/specific names (e.g., tucunaré paca) or a general name (tucunaré). In this case, we considered the values recorded for the general name plus the values for the detailed/specific name. We considered the maximum size of individuals collected in our fish samples (
Silvano 2020) as an indicator of the size of the studied fish species.
We carried out all correlation analyses separately for each river and considered fish species as sampling units. PCA was done using the PRIMER 6 software program (
Clarke and Gorley 2006), and all other analyses used Bioestat software (
Ayres Manuel 2007) and plotted the graphs in R (R Core Team 2013).
Results
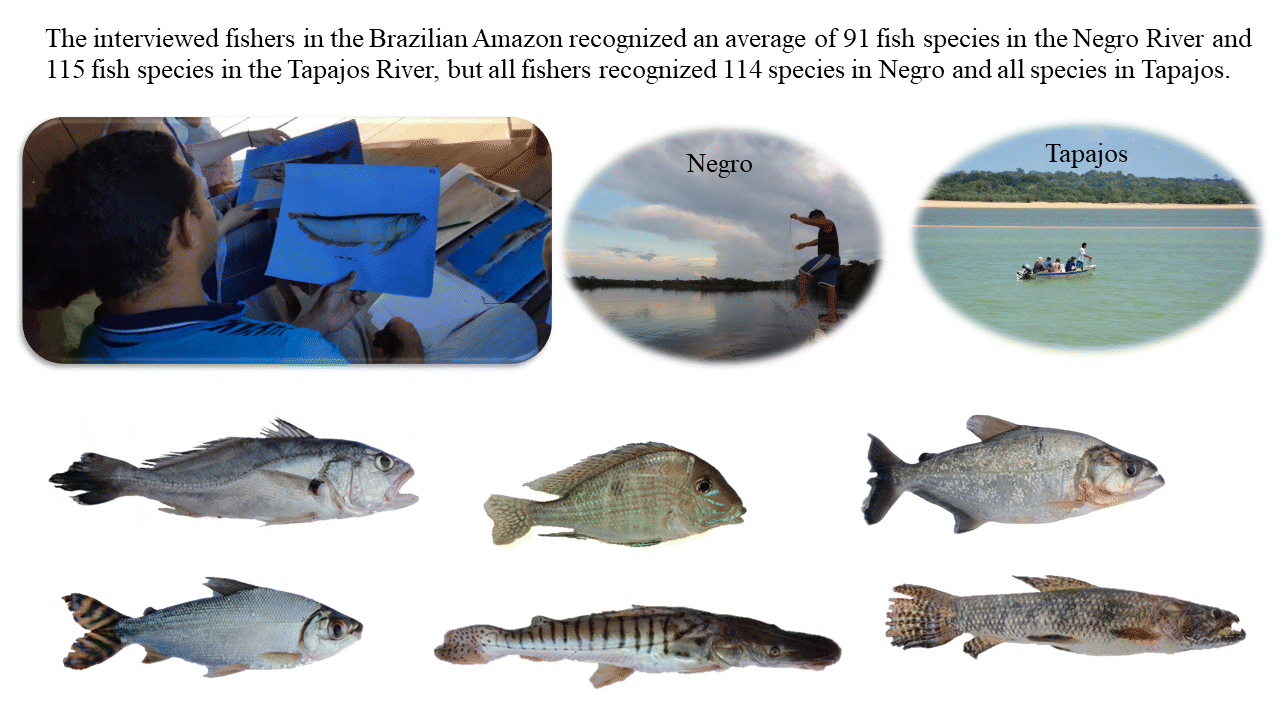
We recorded a total of 231 common names for 115 fish species in the Negro River (
Supplementary Table S1) and 290 common names for 119 species in the Tapajos River (
Supplementary Table S2). The focal species received between one and seven names in both rivers (
Supplementary Tables S1 and
S2) with an average of 2.8 ± 1.4 names in Negro and 3.8 ± 1.6 names in the Tapajos. The average number of names mentioned by the interviewed fishers did not differ between those species recognized by all fishers (median = 2 names, n = 51 species) and species that were not recognized by one or more fishers (median = 3 names, n = 64 species) in the Negro River (
U = 1409.5,
p = 0.21), whereas in the Tapajos River those species that were not recognized by one or more fishers received more names (median = 4.5 names, n = 24 species) than species recognized by all fishers (median = 3 names, n = 95 species,
U = 710,
p < 0.01). The interviewed fishers recognized between 79 and 105 fish species in the Negro (average of 91 ± 10.4 species) and between 98 and 119 (all) species in the Tapajos (average of 115 ± 7.2 species) and could provide distinct names for an average of 67 ± 7.3 species in Negro and 84 ± 11.4 species in Tapajos (
Table 1), as some species received the same name given to other species (
Supplementary Tables S1 and
S2). Therefore, the interviewed fishers recognized, on average, 79% and 97% of fish from photographs shown in the Negro and Tapajos Rivers, respectively, and named more than half of the fish species in both rivers (
Table 1). However, because distinct fish species were recognized by distinct fishers, the total of citations from all fishers indicated that all fish species were recognized in the Tapajos River and 114 species (all except one species) were recognized in the Negro River.
The KI ranged from 1 to −1 in the Negro (
Supplementary Table S1) and from 1 to −0.13 in the Tapajos (
Supplementary Table S2). The distribution of KI values showed a median of 0.38, with limits of upper quartile (75% higher values) of 0.78 and lower quartile (25% lower values) of 0, in the Negro and a median of 0.5, upper quartile of 0.75 and lower quartile of 0.38, in the Tapajos. We thus considered that values of KI equal or higher than the upper quartile would indicate very good recognition of the fish species by the interviewed fishers, values higher than the median would indicate good recognition, values equal to or lower than the median and equal to or higher than the lower quartile would indicate fair recognition, and values lower than the lower quartile would indicate poor recognition. Fishers showed from very good to reasonable (fair) recognition of a total of 88 and 96 fish species in the Negro and Tapajos Rivers, respectively (
Table 2), corresponding to nearly two-thirds of the studied species. Considering the overall recognition patterns, all fish species were recognized by more than half of the interviewed fishers in the Tapajos, whereas in Negro River only 13 species were recognized by less than half of the interviewed fishers and only one species (
Hemiodus atranalis, KI = −1,
Supplementary Table S1) was not recognized by any of the interviewees (
Table 2). The number of species well recognized by the interviewed fishers (very good and good recognition) was similar in the two rivers: 54 species in the Negro and 56 species in the Tapajos, corresponding to 47% of the studied species in both rivers (
Table 2). All interviewed fishers could distinguish 92 and 82 fish species from all other species in the Negro and Tapajos Rivers, respectively; 15 species in the Tapajos and 1 species in Negro were not distinguished by more than half of the interviewed fishers (
Table 2). Therefore, even considering that some fishers gave the same name for more than one species, most fishers mentioned these species to be distinct from one another.
The KI was positively related to fish size (rs = 0.37,
p < 0.0001,
n = 115) (
Fig. 1a), FI (rs = 0.59,
p < 0.0001,
n = 115) representing fishing importance (
Fig. 1b) and AI (rs = 0.4,
p < 0.0001,
n = 115) representing fish abundance (
Fig. 1c) of fish species in the Negro river, whereas in the Tapajos River the KI was positively related to fish size (rs = 0.45,
p < 0.0001,
n = 119) (
Fig. 2) but unrelated to FI (rs = 0.15,
p = 0.12,
n = 119) and AI (rs = 0.06,
p = 0.53,
n = 119). The FI was positively related to AI in the Negro River (rs = 0.36,
p < 0.0001,
n = 115), suggesting that fishers’ perceptions on fish abundance were related to perceptions on importance to fishing, but FI and AI were not related in the Tapajos River (rs = 0.18,
p = 0.06,
n = 119).
The fishers’ perceptions on importance to fishing (FI) were positively related to both the total biomass caught (rs = 0.75,
p < 0.0001,
n = 115,
Fig. 3a) and frequency (rs = 0.77,
p < 0.0001,
n = 115,
Supplementary Fig. S3a) of each fish species on fish landings in the Negro River. The FI was also positively related to both biomass (rs = 0.74,
p < 0.0001,
n = 119,
Fig. 3b) and frequency (rs = 0.77,
p < 0.0001,
n = 119,
Supplementary Fig. 3Sb) of fish caught in the fisheries in the Tapajos River. Nevertheless, the fishers’ perceptions on fish abundance (AI) were unrelated to the biomass of each species sampled in the Negro (rs = 0.13,
p = 0.16,
n = 115) and in the Tapajos (rs = 0.1,
p = 0.3,
n = 119).
The PCA analyses showed 40.5% and 70.6% of explained variation in axis 1 in the Negro (
Supplementary Fig. S4a) and Tapajos (
Supplementary Fig. S4b), respectively. This axis differentiated between most species being mentioned as occurring in the riverside (near the river margin) and lakes in the Negro River (
Supplementary Fig. S4a), while in the Tapajos this axis indicated species occurring in a gradient from lakes and floodplain to the river (
Supplementary Fig. S4b). Overall, most species are considered to occur in the riverine (lotic) habitat in both rivers (
Supplementary Figs. S4a S4b).
Discussion
The fishers we interviewed could recognize and identify (assign a name) to most of the fish species in both rivers, including some species that are not used or that may be rare or small in size. Therefore, fishers know and differentiate more fish species than those 30–40 species currently exploited in fisheries in both rivers (
Hallwass et al. 2020a). Similarly, another study along the coast of Brazil has shown the relevance of fisher’s knowledge on target species to filling information gaps about these species where biological information is scarce (
Begossi et al. 2016).
One limitation of this study is the restricted sampling of only one fisher per community due to logistical constraints related to the duration of the interview, the expertise required from interviewees, and the posterior analyses of a large amount of interview data. We recognize that this sample size does not allow us to check within community variation on fish names or recurrent names consistently assigned to some fish species through binomials (
Begossi et al. 2008). A large sample size could also allow a better evaluation of correspondences between local names (ethnospecies) and scientific names (
Previero et al. 2013;
Pinto et al. 2016). However, despite this limited sample size, the interviewed fishers still recognized and named most fish species in the Negro River and all fish species in the Tapajos River, thus even a few experienced fishers could provide useful and detailed information on fish biodiversity. Furthermore, the rich knowledge of fish names by the interviewed fishers observed in this study reinforces the findings of our previous studies in both rivers, which show the fishers’ detailed knowledge of several aspects of fish ecology, including diet and trophic interactions (
Silvano et al. 2008;
Silvano and Begossi 2016;
Pereyra et al. 2021), migrations (
Nunes et al. 2019), and long-term changes on abundance (
Hallwass et al. 2020b).
The number and overall proportion of species that were well known by fishers were remarkably similar between the two rivers, notwithstanding some environmental differences (a clear and a black water river, located in distinct Amazonian regions) and the lack of contact between fishers from each river. This suggests that some species or groups of species may share distinctive features related to abundance, size, specific characteristics, or importance to fisheries, which are better recognized by fishers in both rivers. The earlier naturalists, such as Wallace in 1848 (
Knapp 2013) and Burkhardt in 1866 (
Britski et al. 2019), who have travelled along the Amazonian rivers, including the Negro, represented in their drawings some of the fish species with high KI (higher than 0.5), reflecting the salience of these fish to these earlier Amazonian explorers. For example, the drawings made by Wallace from his trip to the Negro River include
Cyphocharax abramoides (locally branquinha),
Cichla species,
Osteoglossum bicirrhosum, Leporinus fasciatus, Acestrorhynchus microlepis, Agoniates halecinus, among others (
Knapp 2013), whereas Burkhardt produced 2000 images of fish, including
Pseudoplatystoma tigrinum (
Britski et al. 2019).
A previous study indicated that fishers from other sites in the Negro River identify fish species in more detail compared to Brazilian coastal fishers (
Begossi et al. 2008). This is because Amazonian fishers apply binomials (a generic name plus a specific one) more often to distinguish among species, which could be related either to a higher diversity at the species level in the Amazonian rivers or to a more elaborate knowledge by Amazonian fishers, which reveal fishers’ perceptions on fish morphological or ecological features (
Begossi et al. 2008).
A similar pattern was observed in the Tocantins River, where fishers used binomials to name fish species (
Begossi and Garavello 1990). Interestingly, fishers in the Tocantins River showed a fuzzy recognition and nomination patterns of some fish species from the Loricariidae, as this family include species with difficult recognition and classification criteria in the biological taxonomy (
Begossi and Garavello 1990). The large set of fish species analyzed here confirmed the detailed knowledge about fish ecology and diversity that Amazonian fishers have, thus reinforcing the claims that fishers could act as “parataxonomists” to support rapid biodiversity assessments (
Begossi et al. 2008).
Our overall results suggested that fishers have better recognition of fish species in the Tapajos River compared to fishers in Negro River. This difference is difficult to explain, but it could be related to a higher reliance on commercial fisheries (target species) in the Tapajos, as in Negro, fishers from communities within the RESEX Unini (
Supplementary Fig. S1) are not allowed to sell the fish caught outside the RESEX boundaries (
Hallwass et al. 2020a). Indeed, it seems that more species were recognized in the Negro River by fishers from three communities dedicated to commercial fisheries and located outside the RESEX (Aracari, Bom Jesus, and Aturia,
Table 1).
The number of names attributed to organisms are sometimes considered to be positively related to people’s ethnobiological knowledge, for example about medicinal plants (
Vandebroek et al. 2008;
Vandebroek and Balick 2012). However,
Begossi et al. (2008) observed a positive relationship between the number of names cited by fishers and the number of doubts (when fishers mentioned not knowing the fish) about fish species in the Brazilian coast and in the Negro River (Brazilian Amazon), which indicates that more names cited and less consensus may reflect more confusion and less knowledge. In this study, the number of names cited by fishers did not differ between fish species known by all fishers and those species not known by at least one fisher in the Negro River, whereas in the Tapajos the less recognized species received more names on average. These results indicate that besides reflecting local and regional variations, more cited names may be related to a greater difficulty to recognize a given fish species (
Begossi et al. 2008). The observed averages of 2.8 and 3.8 names per species provided by fishers in Negro and Tapajos Rivers, respectively, were similar to the mean of 3.17 names for shark species (
Carvalho et al. 2018), higher than the average of 1.5 names per fish species (
Previero et al. 2013;
Pinto et al. 2016), but lower than the average of 6 names per fish species (
Freire and Pauly 2005) observed in previous studies with Brazilian coastal fishers. The observed variety of fish names in this study can be at least partially due to regional variations among the studied communities, considering the extension of the studied region (more than 100 km,
Supplementary Figs. S1 and
S2). Indeed, the fish
Hypoclinemus mentalis (
Supplementary Fig. S5) received the name of aramaça by fishers in the first four communities from the Tapajos River’s mouth, whereas its name changed to suia in the next four communities located further upstream, evidencing a geographical variation of names attributed to this species. Therefore, sometimes the lack of consensus on fish names may not necessarily mean reduced knowledge about fish, but may indicate geographical variation.
Two other observations highlight the detailed knowledge of the interviewed fishers. We mistakenly included the large catfish
Brachyplatystoma capapretum (
Supplementary Fig. S6a) in the set of photos to be shown to fishers in the Tapajos, but this fish species does not occur in this river, where a similar species of the same genera,
B. filamentosum (
Supplementary Fig. S6b) can be found and which is indeed important to fisheries (
Nunes et al. 2019,
Hallwass 2020a,
2020b;
Silvano 2020). However, we maintained the data for this species in our analysis (
Table S2), as the interviewed fishers noticed the difference in the photograph shown and, although they provided information for the related species (filhote,
Table S2), they alerted us that the fish shown is not usually found in the Tapajos River. In the Negro River, we included two photographs of the species
Myloplus nigrolineatus (pacu galo,
Table S1), which has morphological differences between females (
Fig. S7a) and males (
Fig. S7b) (
Ota et al. 2020,
Silvano 2020). Nevertheless, at least half of the interviewed fishers in the Negro River accurately mentioned that these two fish would be female and male of the same species (the same kind of fish), so we pooled the data of these two photographs for the analyses.
Although much simpler than the more elaborated knowledge indexes proposed in ethnobotany (
Sousa Araújo et al. 2012), the KI adopted here allowed quantitative analyses and comparisons to be made regarding fishers’ knowledge on a large set of fish species. This simple index and the approach adopted in this study could be thus widely applied to evaluate fishers’ knowledge on fish diversity in other regions of the Amazon and elsewhere, including other tropical river basins with high fish diversity, but lacking scientific information and threatened by development projects, such as those in Southeast Asia and Africa (
Winemiller et al. 2016;
Baird et al. 2021).
The knowledge (KI) held by fishers was positively related to fishers’ perceptions on fish species abundance, size and importance to fisheries in the Negro River, but the KI was only related to fish size in the Tapajos River. This further confirm that fishers’ knowledge about fish species are related to multiple features, including either the fish usefulness to fisheries (
Begossi and De Figueiredo 1995;
Begossi et al. 2008;
Carvalho et al. 2018), or characteristics that makes fish more noticeable to fishers (salience), such as abundance and size (
Hunn 1999). Indeed, according to
Hunn (1999), fish size significantly affects salience, as “the larger the animal, the finer the degree of taxonomic differentiation” (p. 52). Moreover, those fishes readily recognized (by more than 80% of fishers) in the pictures shown to fishers in a previous study were also species with high KI in this study, such as species of
Brachyplatystoma,
Brycon,
Cichla,
Leporinus,
Myloplus,
Serrasalmus and
Osteoglossum bicirrhosum, among others (
Begossi et al. 2008). Fishers also formed fish groups (“relatives”, cousins) that corresponds to taxonomic families, especially with species of relative high KI (
Begossi et al. 2008).
The fishers’ perceptions on importance to fisheries was related to data from fisheries monitoring in both rivers and at the same communities (
Hallwass et al. 2020a). A previous study similarly shows a positive relation between fishers’ citations and fisheries data for fish species in the Tocantins River, also in the Brazilian Amazon (
Hallwass et al. 2013a). In the absence of fisheries monitoring, interviews with fishers have been widely applied to gather quantitative data on fish use, catches and consumption in the Brazilian Amazon (
Begossi et al. 1999,
2019;
Isaac et al. 2015;
Hallwass et al. 2020b;
Runde et al. 2020) and in other tropical rivers (
Fluet-Chouinard et al. 2018). Our results further support the applicability of interviews with fishers to gather needed data on the use and cultural relevance of fish species in high diversity aquatic ecosystems. The detailed studies on fish local names can improve fisheries monitoring systems (
Freire and Pauly 2005;
Previero et al. 2013) and the assessment of fishing effects on distinct fish species to develop proper management actions (
Carvalho et al. 2018). Therefore, the observed fishers’ knowledge on fish species names and their relative importance to fishing in the studied rivers can stimulate and improve initiatives of participatory monitoring of small-scale fisheries in Amazonian rivers (
Silvano and Hallwass 2020).
Notwithstanding their overall good knowledge on most of the fish species, fishers’ perceptions regarding fish abundance were unrelated to the relative abundance of each species assessed through biological sampling in both rivers (
Silvano 2020). These disagreements between fishers’ local ecological knowledge and biological studies do not necessarily mean that fishers would be equivocated (
Silvano and Valbo-Jørgensen 2008). This indicates possible uncertainty and variation of estimates from both fishers’ knowledge and biological sampling. For example, standardized biological sampling may underrepresent the abundance of some fish species exploited by fishers, who use more selective fishing gear (
Hallwass and Silvano 2016). On the other hand, fishers may overestimate the abundance of some fish species, especially those more valuable or usually harvested (
Hallwass et al. 2013a). Another possibility is a mismatch in scale, as fishers’ answers are more related to fish abundance in the area surrounding their communities, while fish sampling data were pooled for 8 fish samples in each river, including all the studied communities. Therefore, both sources of information may complement each other.
The interviewed fishers also provided information on habitat use by the focal fish species. The river and riverside (close to shore or river’s margin) were the main habitats cited for most of the fishes in both rivers. The stretch of Tapajos River studied may have a lower heterogeneity of habitats, since the river channel is wide (10–15 km,
Fig. S2) with a narrow floodplain area and fisheries occur mainly (more than 70% of fish landings) in the river channel (
Hallwass et al. 2020a). On the other hand, Negro River has a larger floodplain and possibly more heterogeneous habitats, and fisheries are more homogeneously distributed among aquatic habitats (
Hallwass et al. 2020a). This may had influenced a higher variety of habitat information from fishers in the Negro river. Despite the aforementioned differences between the two rivers, we observed some similarities in habitats used by some fish species according to fishers. For example, the jacundá (
Crenicichla spp.,
Tables S1 and
S2) was associated to the floodplain in both rivers. The combined data from fishers’ knowledge on species recognition, use (fishing) and habitat could be applied in rapid environmental impact assessments over a broad geographical scale, based on interviews with fishers. For example, fishers’ knowledge can considerably improve the assessment of downstream impacts from existing or proposed dams, especially in less studied tropical rivers (
Baird et al. 2021), such as Amazonian rivers (
Hallwass et al. 2013a;
Santos et al. 2020), including the Tapajos (
Nunes et al. 2019;
Runde et al. 2020). The methodological approach adopted in this study allows fishers and researchers to quickly recognize the fish species occurring in an area and how these species are used by local people. This simple and straightforward methodology could be broadly replicated throughout the Brazilian Amazon, for example by using available books with photos of fish species from many Amazonian rivers (
Ferreira et al. 1998;
Silvano 2001;
Santos et al. 2004;
Ohara et al. 2017) including the Tapajos and Negro rivers (
Silvano 2020).