Introduction
Biodiversity is declining globally and increasing numbers of species are being threatened with extinction (
Ceballos et al. 2015;
Reid et al. 2019). To determine reasons for species decline and help prioritize conservation efforts, multiple organizations assess and list imperiled species (
Possingham et al. 2002). Species listings often accompany legislation which requires the development of species recovery plans to direct management actions (e.g., the US
Endangered Species Conservation Act 1973; Canadian
Species at Risk Act 2002). While recovery planning can help curb further decline of imperiled species (
Taylor et al. 2005), the effectiveness of single and multispecies recovery plans depend on the connection among species, the biological processes involved in recovery, and the effective restoration of their habitats (
Dee Boersma et al. 2001;
Clark and Harvey 2002). Changes in environmental conditions are often contributing causes of species decline (
Reid et al. 2019) but the failure of the “field of dreams” hypotheses suggests that restoring habitat alone is insufficient (
Palmer et al. 2010). As such, understanding the extent to which the persistence of co-occurring species is supported through both positive (e.g., mutualisms, facilitation) and negative (e.g., predation, competition) interactions (
Wisz et al. 2013) is necessary to more effectively manage imperiled species. That is, species interactions management may be necessary to ensure successful species recovery and so may require innovative habitat restoration approaches (
Stewart et al. 2022).
The nature of species co-occurrence and interspecific interactions offer critical mechanistic insight to guide species recovery (
Jones et al. 2018). In practice, biotic interactions appear to be better addressed in terrestrial ecosystem restoration (
Wainwright et al. 2018), whereas aquatic efforts often focus on abiotic conditions (
Bond and Lake 2003;
Palmer et al. 2010) despite growing appreciation that managing for biotic interactions can improve restoration outcomes (
Lake et al. 2007;
Barrett et al. 2021). Specifically, manipulating interactions (e.g., mutualisms, cross-ecosystem subsidies;
Halpern et al. 2007) likely aids or accelerates species recovery. Species assemblage information can inform the selection of focal species to act as intermediate indicators of recovery, or act as umbrella species for multiple species (
Kalinkat et al. 2017). As such, accounting for interactions between common and imperiled species across a variety of guilds offers a promising avenue to explore to improve upon in species recovery efforts.
Across freshwater ecosystems globally, benthic macroinvertebrates are a common indicator used in habitat restoration and ecosystem health monitoring (
Buss et al. 2015;
Eriksen et al. 2021). Among this group, freshwater unionid mussels (mussel, hereafter) are distinct in their reliance on interspecific interactions to complete their life cycle and provide supportive ecosystem functions for other species (
Modesto et al. 2018;
Vaughn 2018). Existing assessments of mussel restoration have largely focused on host fish–mussel relationships (e.g., propagation and relocation;
Patterson et al. 2018), while other species interactions are largely missing from the published literature (
Eveleens and Febria 2022). The evaluation of factors driving mussel species assemblages has for the most part focused on environmental variables alone (
Atkinson et al. 2012;
Chambers and Woolnough 2018) or the role of host fishes with limited consideration for environmental conditions (
Schwalb et al. 2013). However, the ecological functions generated by mussels may facilitate positive feedback loops supporting other mussel species presence and abundance (
Atkinson et al. 2018). In practice, this may translate to the recovery of supporting and/or common species in tandem with focal species.
In parallel, species recovery is not possible without consideration of habitat conditions and anthropogenic pressures themselves. Stressors causing declines in mussel diversity, such as eutrophication and sedimentation (
Gascho Landis et al. 2013;
Lopes-Lima et al. 2018) also have predictable adverse affects on benthic macroinvertebrate diversity and abundance (
Burdon et al. 2013). Given that benthic macroinvertebrates benefit from mussel-derived nutrient subsidies (
Spooner and Vaughn 2006) and habitat enhancement (
Beckett et al. 1996), it is logical to assume that the effects of human-derived stressors on both species groups are linked. As such, the sampling of benthic macroinvertebrates could offer a complementary approach to assess mussel communities where nonmussel macroinvertebrate assemblages respond more rapidly to environmental change, require less field sampling effort, and have an established knowledge base on tolerance levels of taxa to specific stressors. Alternatively, sampling of mussel communities could offer immediate insight into whole benthic macroinvertebrate community assemblages as mussel surveys are for the most part completed in situ and do not require the laboratory processing of benthic samples. The reality is that species protection and watershed monitoring are often managed by different entities (e.g., federal agencies and local conservation authorities, respectively), thus, the triangulation of species recovery, watershed health monitoring, and habitat restoration have been untested or underexplored.
We explored this knowledge gap by investigating mussel species co-occurrence within mussel communities and the broader macroinvertebrate communities across a biodiverse river system in southern Ontario, Canada. Data sets from local and federal organizations (comprising watershed biomonitoring and mussel records, respectively) were combined with a novel field survey to examine relationships between the total mussel community (species presence and richness) and the presence and richness of mussel species at risk (SAR) and/or benthic macroinvertebrates other than mussels. We hypothesized that (1) higher total mussel species richness would correlate with the presence of listed SAR, and (2) mussel community composition (species presence and richness) would differ significantly among sites where listed SAR are present and where they are not. Additionally, we posed that (3) higher mussel species richness and the number of SAR present would correlate with greater taxonomic richness of benthic macroinvertebrate communities.
Methods
Mussel and benthic macroinvertebrate data were collected from the Sydenham River watershed of southwestern Ontario, Canada. Situated in the Laurentian Great Lakes basin (Nayaano-nibiimaang Gichigamiin in Anishnaabemowin) and the Traditional Territory of the many First Nations including the Three Fires Confederacy of First Nations (the Odawa, the Ojibwe, and the Potawatami), the Mississaugas and Attawateron (Neutral), this watershed supports diverse freshwater fauna including 35 unionid mussel species (
McNichols-O’Rourke et al. 2012). Of these unionid species, 15 are federally listed SAR under the Canadian
Species at Risk Act 2002 (
Environment and Climate Change Canada 2021;
Species at Risk Act 2002). Nine SAR found in the Sydenham River are classified as Endangered, two are classified as Threatened species, and the remaining four are listed as Special Concern (
Environment and Climate Change Canada 2021). No other benthic macroinvertebrates found in the Sydenham River are federally listed. Study site selection was informed by the availability of federal and regional biomonitoring data, including mussel, benthic macroinvertebrate, and habitat data. The limited overlap between the separate mussel and macroinvertebrate data sets necessitated an additional field survey to harmonize the data sets and facilitate testing of our hypotheses.
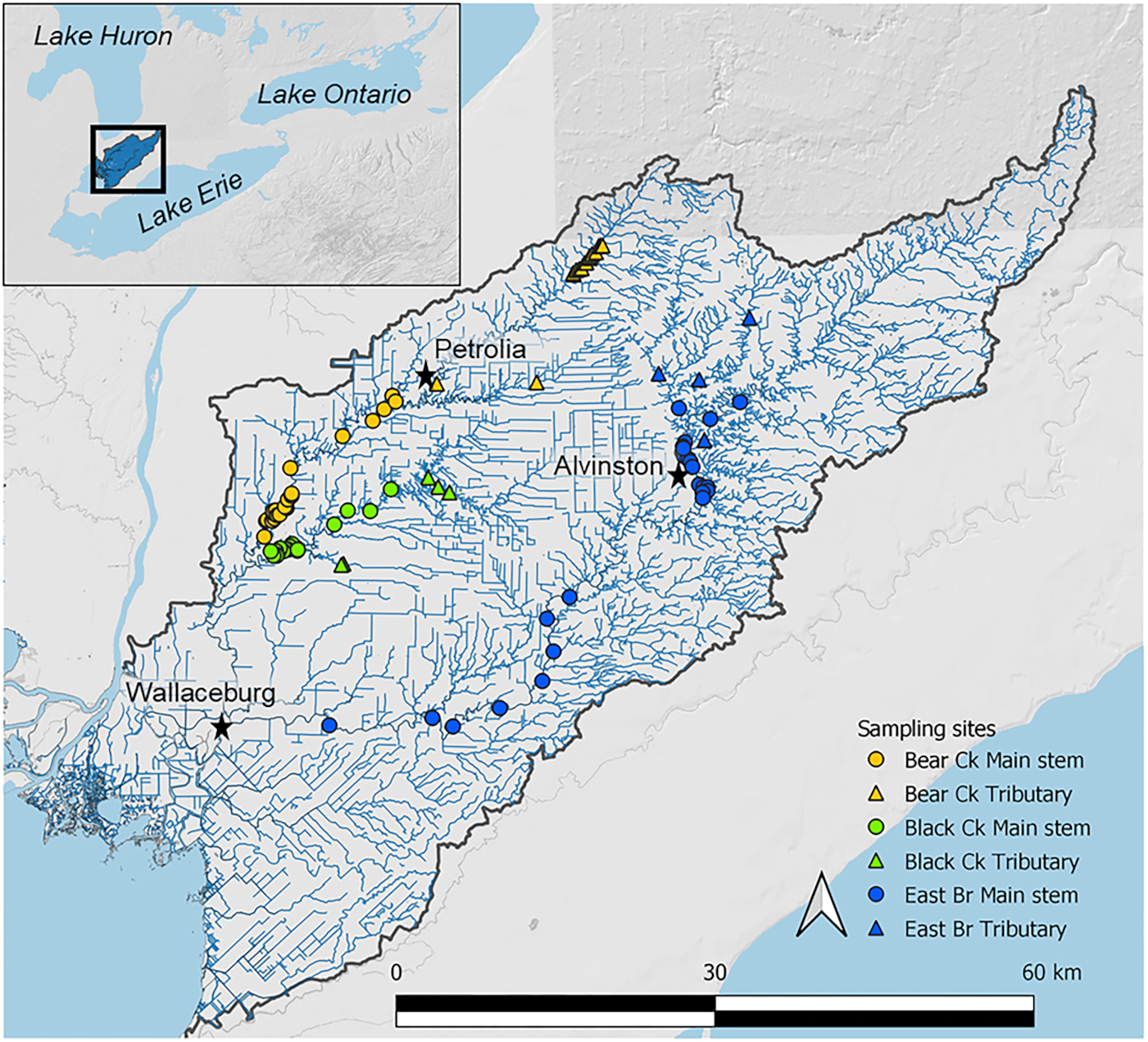
The data sets analysed include existing mussel survey records obtained from Fisheries and Oceans Canada (DFO; T.J. Morris (unpublished data)) and St. Clair Region Conservation Authority (SCRCA; E. Carroll, (unpublished data)) spanning from 2012 to 2018, as well as field survey data collected in August 2020. The existing DFO and SCRCA mussel records and mussel data from the field survey were combined into a single data set solely containing mussel species presence/absence data (hereafter “the combined data set”), which included 79 existing sites from DFO and SCRCA, and a further 15 sites from the 2020 field survey (total
n = 94,
Fig. 1, Supplementary Information (SI) Table S1). Given that multiple survey protocols were employed across agencies, it was not possible to include a comparable measure of abundance in the combined data set. The field survey also collected benthic macroinvertebrate and environmental data to match mussel data from the same 15 sites—hereafter, referred to as “field survey data” (sites listed in SI Table S2). Macroinvertebrate and environmental data were sampled once at each of 15 sites in 2020. Sites were located following a stratified random approach across the three main subwatersheds—nine sites in the East Branch, three sites in Black Creek, and three sites in Bear Creek to ensure a gradient of mussel diversity was sampled (see Supplementary Methods for further detail). Existing DFO and SCRCA mussel data, collected with the same protocol, were used for 5 sites with new collections made at the remaining 10 sites (see SI Table S2). Five sites were identified as tributary sites (four on the East Branch, one on Black Creek) while all other sites were considered main stem.
Data collection
A hybrid protocol combining timed and quadrat searches was employed for mussel surveys to ensure the detection of rare species, match existing protocols, and increase sampling efficiency (
Villella and Smith 2005;
Ring and Woolnough 2022). While timed searches are commonly used for surveying mussels, quadrat surveys are the standard federal monitoring approach in Canada because they better capture the species present, detect smaller mussels more effectively and permit assessment of reproduction (
Metcalfe-Smith et al. 2000;
Reid and Morris 2017). The two approaches were combined to ensure robust data collection at sites where mussels were present, while also minimizing time investment at sites with depauperate mussel communities. At all sites, a 4.5 person hour timed search was completed following the standard protocol from
Metcalfe-Smith et al. (2000). If either >25 live individuals or a live SAR were located, an additional quadrat survey was also completed. Quadrats were located prior to the timed search and were excluded from the timed search area to ensure that quadrat results were not confounded by the timed search when required. Ten 1 m × 1 m square quadrats were excavated to a substrate depth of 15 cm, or until bedrock or clay hardpan was reached (
Metcalfe-Smith et al. 2007). Quadrats were randomly located within the survey reach using a triple randomized process of randomizing the distance from the most upstream end of the site, the distance off the river centre line, and placement of quadrats left or right off the centre line (
Sheldon et al. 2018). Due to high turbidity, tactile sampling was employed for the majority of sampling (
Metcalfe-Smith et al. 2000;
Mackie et al. 2008). Each mussel found alive during both survey methods was identified, photographed, and counted, before being immediately returned to the river. Mussel species names followed nomenclature set out by the MolluscaBase taxonomic database (
MolluscaBase 2021) and identification followed
Metcalfe-Smith et al. (2005) with additional expert advice sought to clarify any identification uncertainties when needed. Both reach (<500 m, defined as 20× bankfull width) and quadrat-scale environmental data were collected during mussel and benthic macroinvertebrate surveys including water chemistry, channel morphology, substrate classification, discharge, flow velocity, adjacent vegetation, and nutrient concentrations. Collection methods for environmental data predominantly followed Ontario Stream Assessment Protocols (
Stanfield et al. 2017, see Supplementary Methods).
Benthic macroinvertebrates samples were collected from three locations per site using a timed kicknet in September 2020. Three standardized 3 min traveling kicknet samples per site were taken moving perpendicular to the direction of flow using a 500 µm mesh net. This followed Ontario Benthic Biomonitoring Network (OBBN) protocols to ensure consistency with existing biomonitoring data sets (
Jones et al. 2007). Macroinvertebrate samples were collected from the sampling reaches surveyed for mussels at least 2 weeks after mussel surveys, with samples taken across a riffle–pool–riffle sequence or one meander wavelength if distinct riffle–pool sequences were not present (
Jones et al. 2007). All samples were immediately preserved in formal ethanol (15 parts 95% ethanol, 3 parts 37% formaldehyde, and 7 g Borax per 1 L volume;
Krogmann and Holstein 2010;
Dumke et al. 2013) and were laboratory processed under 10× magnification using a Leica dissection microscope. All individuals were identified to family or lower using keys from
Peckarsky et al. (1990) and
Merritt et al. (2008) and counted.
Analyses
To assess whether higher total mussel species richness correlated with the presence of SAR, the relationship between total mussel species richness and SAR species richness was evaluated for the combined data set using generalized linear models with a Poisson error distribution to account for the use of count data. Analyses were also repeated only including species listed as Endangered and Threatened to investigate if species patterns could assist in informing specific federal requirements for the protection of Endangered and Threatened species, including the creation of recovery plans and protection of critical habitat (
Species at Risk Act 2002).
To assess the variation of overall mussel community composition among sites where listed SAR were present and where they were not, a Nonmetric Multidimensional Scaling ordination was run for the combined data set using Bray–Curtis dissimilarity distance (
Faith et al. 1987;
Christian et al. 2021). The significance of differences in mussel community composition between sites where SAR were present and where they were absent was evaluated using a permutational analysis of variance (PERMANOVA) with 999 permutations. Differences in community composition between where Endangered and Threatened species were found, where just species of Special Concern, and where no SAR were found were assessed using the same approach.
Patterns between benthic macroinvertebrate communities and mussel community characteristics were examined by comparing benthic macroinvertebrate diversity metrics to mussel species richness and SAR presence from the field survey data. Benthic macroinvertebrate metrics calculated and assessed included overall benthic macroinvertebrate family richness, the relative abundance of sensitive Ephemeroptera, Plecoptera, and Trichoptera taxa (percent EPT;
Rosenberg and Resh 1993;
Marchant et al. 1995), and the Hilsenhoff Biotic Index (HBI;
Hilsenhoff 1988). Diversity metrics were compared to mussel species richness among sites using generalized linear models employing a Poisson error distribution. Finally, benthic macroinvertebrate taxa indicative of the presence of Endangered or Threatened mussel species were identified using Indicator Taxa Analysis (ITA) involving evaluating the presence/absence of macroinvertebrate taxa against the presence of Endangered or Threatened mussel species found in the field survey (
Dufrêne and Legendre 1997;
Christian et al. 2021).
Variation in environmental conditions among sites was examined using Principal Component Analysis (PCA) of the environmental data obtained by the field survey to determine how environmental conditions varied across sites. To apply multivariate analyses to proportional and categorical data (e.g., substate classification, riparian vegetation cover), Correspondence Analysis (CA) was used to summarize the variation within each group of variables into ≥1 CA axes to account for the nonindependence of individual variables (
Jackson 1997;
Neff and Jackson 2011). The resulting site scores were included in the PCA as new variables to represent variation in channel morphology (C1, C2), riparian vegetation (R1, R2), and substrate composition (SI Table S5). To examine whether environmental conditions varied longitudinally, sites were classed as being on the main stem river or on smaller stream tributaries using Strahler stream order. Sites having a Strahler stream order of ≥5 were classed as main stem sites, while fourth order sites or lower were considered tributaries based on field observations (see Supplementary Methods). All analyses were done in R 3.6.0 (
R Core Team 2020), with multivariate analyses completed using the
vegan package (
Oksanen et al. 2019) and ITA done using the
labdsv package (
Roberts 2019).
Results
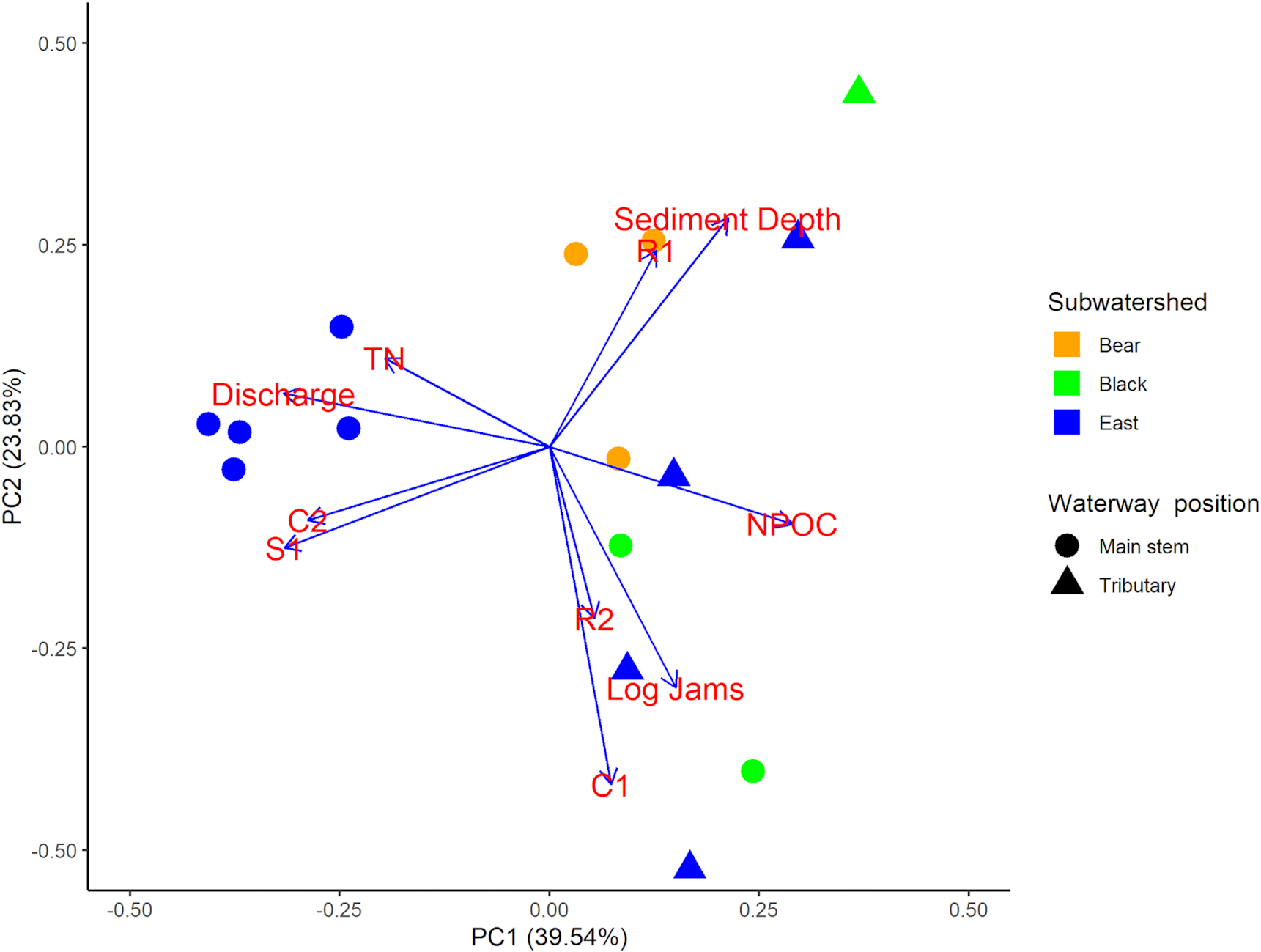
We sampled a total of 15 sites across the Sydenham River watershed in 2020, all of which were selected using a stratified random approach across the three main subwatersheds. The final distribution of sites resulted in nine sites in the East Branch, three sites in Black Creek, and three sites in Bear Creek to ensure a broad gradient potential habitat conditions and mussel diversity (see Supplementary Methods for further detail). Mussel, macroinvertebrate, and environmental data were gathered in alignment with existing DFO and SCRCA mussel data, using comparable protocols (see SI Table S2). In terms of stream order, five sites were identified as tributary sites (four on the East Branch, one on Black creek) while all other sites were considered main stem. In total, 79 existing sites and 15 sites sampled during the field survey were included in final analyses, with 32 mussel species (13 SAR, 9 Endangered, and 2 Threatened species) recorded across all 94 sites in the combined data set. Environmental conditions varied across sites, with differences best described by discharge, total nitrogen, dissolved organic carbon, riparian vegetation, channel morphology and substrate, log jams, and uncompacted sediment depth; 63.37% of environmental variation was described by the first two axes of the PCA (
Fig. 2).Bear and Black Creeks were characterized by predominantly silt and clay substrates with a high proportion of run and pool habitat, as well as less dense riparian vegetation. The East Branch sites had a higher discharge with larger, more rocky substrate and a higher proportion of riffles habitat. Tributaries displayed increased dissolved organic matter concentrations, higher turbidity and conductivity, but lower dissolved oxygen saturation relative to mainstem sites. Seventy-four macroinvertebrate taxa were recorded across the 15 sites included in field survey. At individual sites, mussel species richness ranged from 1 to 25 species (
Table 1), with SAR present at 78 of 94 sites and Endangered and/or Threatened species present at 39. Macroinvertebrate taxa richness spanned from 16 to 39 taxa (
Table 1).
Mussel species richness was significantly higher where SAR were present (Deviance explained = 65.839, Residual deviance = 270.11,
P < 0.001), and where Endangered and/or Threatened mussel species were found (Deviance explained = 187.35, Residual deviance = 148.60,
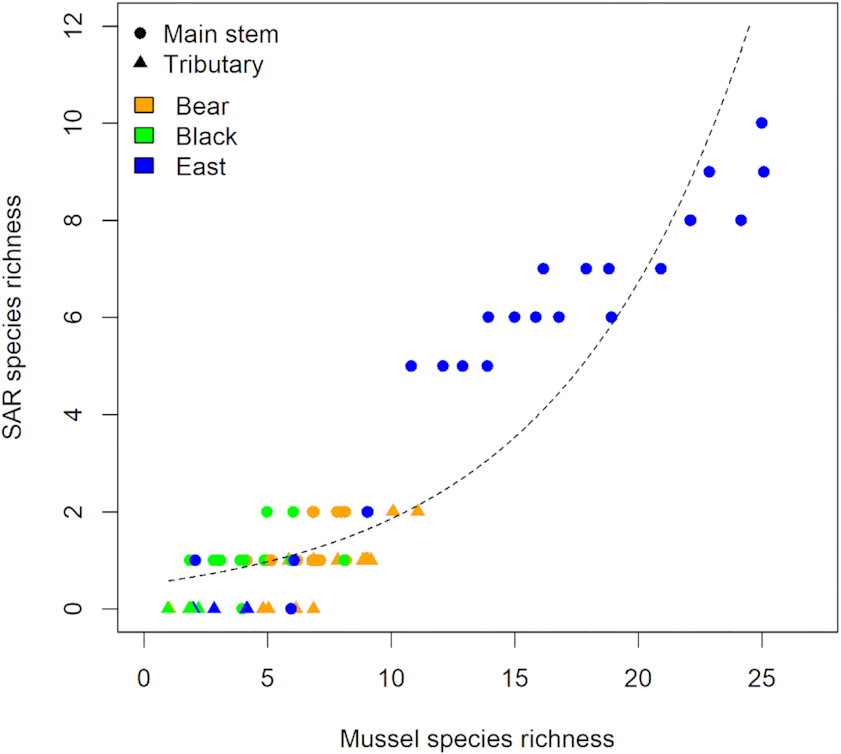
P < 0.001). Moreover, mussel species richness and SAR species richness were positively correlated when analysed using Poisson regression (slope = 0.129,
Fig. 3). Main stem sites in the East Branch of the Sydenham River were found to contain a diverse assembly of 31 species, including all SAR found. In contrast, Bear and Black Creeks had lower species richness and the species that were present are commonly associated with fine-sediment substrates, including
Potamilus fragilis (Fragile Papershell),
Potamilus alatus (Pink Heelsplitter), and
Quadrula quadrula (Mapleleaf). Two species,
Lasmigona complanata (White Heelsplitter) and
Pyganodon grandis (Giant Floater), were present at nearly all sites (Table S6).
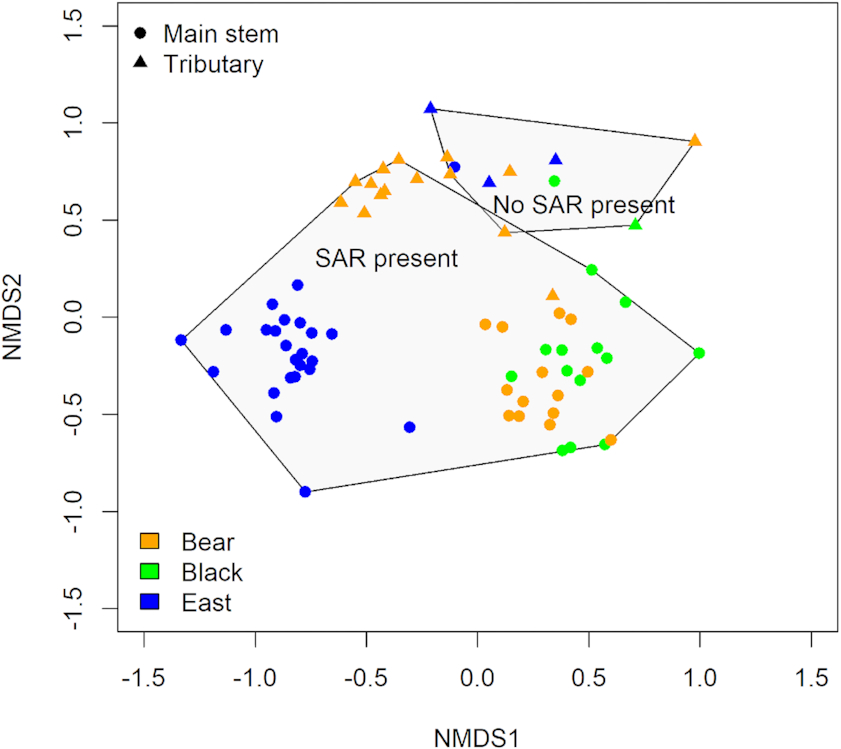
Alongside differences in species richness, mussel community composition differed significantly between sites where SAR were present and/or absent (
Fig. 4, PERMANOVA
F[2,93] = 17.273,
P = 0.001). A significant difference in community composition was also found between where Endangered and/or Threatened species were found compared with other SAR and where no SAR were found (PERMANOVA
F[2,93] = 36.43,
P = 0.001), as communities split into three groupings. Sites with SAR present comprised one group characterized by
Quadrula quadrula,
Pyganodon grandis, and
Truncillia truncata (Deertoe) and another species-rich group of riffle-dwelling species including
Epioblasma rangiana (Northern Riffleshell),
Fusconaia flava (Wabash Pigtoe), and
Truncilla donaciformis (Fawnsfoot). This riffle-dwelling group contained the Endangered and Threatened species while sites with no SAR were characterized by
Anodontoides ferussacianus (Cylindrical Papershell) and
Lampsilis siliquoidea (Fat Mucket).
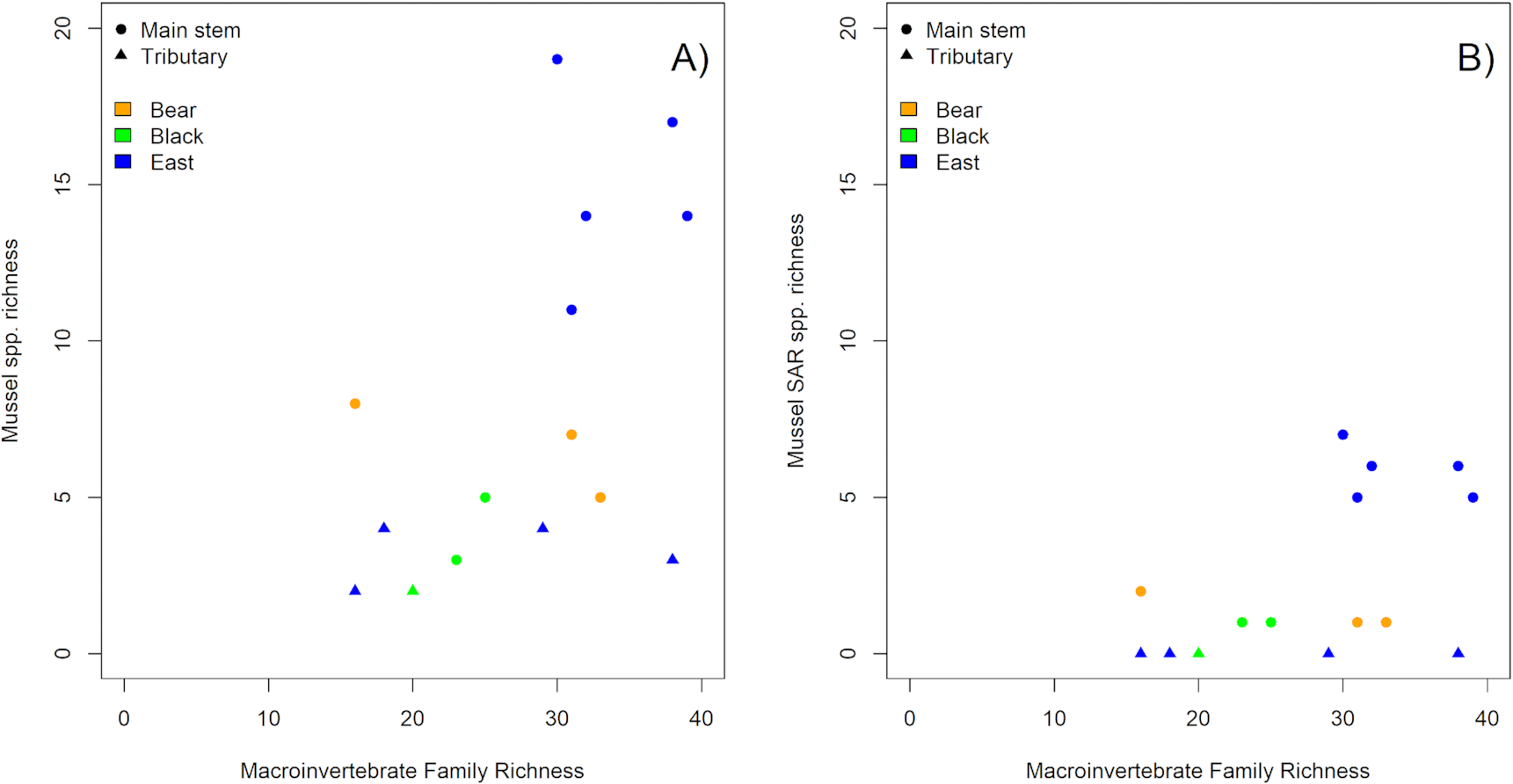
Higher total mussel species richness and the presence of more SAR correlated with greater taxonomic richness of benthic macroinvertebrate communities, although subwatershed and waterway position also had significant effects (Table S7). Benthic macroinvertebrate diversity metrics displayed significant relationships with mussel species richness for the 15 sites surveyed for mussels and benthic macroinvertebrates in the 2020 field survey. Macroinvertebrate family richness (
Fig. 5A; Deviance explained = 16.8489, Residual deviance = 38.740,
P < 0.001), as well as the percent EPT taxa were positively related to mussel species richness (Deviance explained = 13.4393, Residual deviance = 42.150,
P < 0.001; Table S7). Similarly, HBI scores were related to mussel species richness, with lower HBI scores (indicating greater ecosystem health) correlating with higher mussel species richness (Deviance explained = 11.4355, Residual deviance = 44.153,
P < 0.001; Table S7). The same significant relationships held true for the occurrence of SAR (
Fig. 5B, Table S8), and for Endangered and/or Threatened species (Table S9). Indicator Taxa Analyses (ITAs) showed that, while there were no macroinvertebrate indicator taxa for the presence of mussel SAR, the specific presence of Isonychiidae, Leptohyphidae, Psephenidae, and Simuliidae indicated the likely occurrence of Endangered or Threatened mussel species (
Table 2). When ITA were conducted for individual Endangered or Threatened mussel species, 19 unique indicator taxa for the presence or absence of individual Endangered or Threatened species were identified (
Table 3).
Discussion
This study demonstrated strong alignment across federal and local data sets collected in the same watershed but targeting different species or ecosystem health indicators. When bolstered by a local, targeted survey, we were able to generate insight into the relationships within and between mussel communities and the broader benthic macroinvertebrate community. For the first time, we showed that mussel species richness and the occurrence of mussel SAR were positively related, that the presence of SAR matches overall mussel community composition, and that both mussel species richness and the presence of SAR correspond to commonly employed benthic macroinvertebrates diversity indices. Relating mussel and macroinvertebrate diversity, as well as identifying specific indicator species for Endangered and Threatened species allow us to pose that macroinvertebrate community composition could inform the selection of suitable mussel habitat for recovery actions.
Leveraging and repurposing existing data sets in this way, together with the strategic inclusion of additional environmental and macroinvertebrate data enabled us to more rigorously evaluate patterns of community composition for mussels and macroinvertebrates than any individual data set or sampling effort alone. Changes in mussel species richness and differences in the number of SAR present across a range of environmental conditions supports existing knowledge that habitat and environmental conditions influence mussel distribution. The observed differences in the mussel species present were consistent with known differences in species habitat preference across substrate types and flow conditions (
McRae et al. 2004;
Morris and Burridge 2006;
Randklev et al. 2019). More diverse mussel communities were found in main stem sites on the East Branch of the Sydenham River, where sites were characterized by more heterogeneous substrate. In contrast, sites in other subwatersheds were represented by fine-sediment and smaller sized substrate (e.g., sand and silt). As such, mussel communities associated with these sites were expected due to known adaptations of species to particular substrate types (
McRae et al. 2004;
Goodding et al. 2019).
SAR occurrences at sites with higher mussel species and benthic macroinvertebrate richness are an example of positive interactions and healthy resilience (sensu
Barrett et al. 2021). However, there is a need to investigate this further across a range of mussel species associations. While hotspots of SAR richness were already known to occur in areas with high overall biodiversity at watershed or subwatershed scales (e.g.,
Staton and Mandrak 2005;
Newton et al. 2008), the relationship between elevated numbers of SAR, the correlation of high mussel species richness and macroinvertebrate diversity had not been previously described at the reach scale (e.g., tributary and main stem). In this study, Endangered and Threatened mussel species were only found in heterogeneous communities with nonlisted species present and never alone. The observation that Endangered or Threatened species occurred as part of distinct mussel communities and were never found to occur alone suggests that Endangered or Threatened species are more vulnerable to environmental stressors, require the presence of other mussels to persist, or a combination of both.
Potentially important biotic drivers of mussel occurrences include food supply, host fish presence, and species interactions (
Newton et al. 2008;
Modesto et al. 2018). The ecosystem functions provided by mussels generally benefit the supply of nutrients across aquatic food webs (
Vaughn 2018) and may facilitate the occurrence of other mussel species through enhancing habitat conditions. In turn, potentially creating positive feedback loops supporting increased local species richness. Such feedback loops could explain the accumulations of Endangered and Threatened species that were observed within the most diverse mussel assemblages because there the potential for beneficial interactions to occur among mussel species has not been quantified (
Vaughn et al. 2008). Studies into ecosystem function show that richer assemblages of mussel species enhance nutrient subsidies (
Allen et al. 2012) and the resilience of nutrient cycling (
Atkinson et al. 2018). In turn, this could drive direct facilitation through increasing resource availability and supporting ecosystem functioning, or provide indirect facilitation by increasing the abundance of host fish through bottom-up trophic cascades (
Firth et al. 2021). Additionally, some rare mussels have been shown to display higher body condition in more species rich communities, suggesting a fitness benefit from the interspecific interactions taking place within richer mussel communities (
Spooner 2007). Therefore, the type and strength of interactions occurring among co-occurring mussel species need to be revisited and consideration given to whether positive species interactions with common mussel species are required to facilitate the persistence of imperiled species; we would hypothesize that this would be the case and the data from this study help support this hypothesis.
Benthic macroinvertebrates diversity indices displayed a positive relationship with both mussel species richness and the occurrence of mussel SAR, although subwatershed and waterway position were equally important predictors of mussel species richness. Benthic macroinvertebrates were expected to display predictable patterns of variation in community assemblage (
Vannote et al. 1980;
Death 1995;
Neff and Jackson 2011), however, the relationship of macroinvertebrate diversity and mussels was unknown. While macroinvertebrate abundance and emergence rates have been shown to benefit from mussel-derived nutrient subsidises (
Spooner and Vaughn 2006;
Allen et al. 2012) and mussels are known to enhance macroinvertebrate habitat (
Beckett et al. 1996), it had not previously been demonstrated whether the composition of both mussel and macroinvertebrate communities displayed similar patterns of assembly. While mussel and macroinvertebrate diversity are known to be driven by environmental conditions (
Townsend and Hildrew 1994;
Atkinson et al. 2012), our results suggest that mussel and macroinvertebrate communities appear to be driven by a similar combination of environmental and community processes. For example, fine sediment is a major stressor to both species groups. Benthic macroinvertebrates are impacted to the degree that sensitive species are eliminated (
Burdon et al. 2013), while fine sediment is known to adversely affect mussel feeding (
Tuttle-Raycraft et al. 2017), as well as reproduction (
Gascho Landis et al. 2013;
Österling 2019). Hence restoration actions that increase overall macroinvertebrate diversity will likely also benefit mussels. Unlike mussels, abundant macroinvertebrate taxa have adult life stages with high dispersal potential and fast generation times that allow populations to respond quickly to environmental change (
Eriksen et al. 2021); mussels have limited options for escape. Therefore, the characteristics that make macroinvertebrates a highly effective biomonitoring tool may be transferrable to indicating mussel species declines or recovery (
Resh 2008) but will require additional measures to ensure mussel populations can thrive (e.g., restoration sequences involving population support or augmentation;
Eveleens and Febria 2022).
Understanding of the extent to which mussel species and macroinvertebrate taxa coexist and interact is essential for species conservation and habitat restoration. The identification of benthic macroinvertebrate indicator taxa for Endangered and Threatened mussel species is a novel finding of this study and offers a new application for existing data sets and monitoring programs (e.g., assisting the identification of critical habitat). Macroinvertebrates as indicators could provide significant utility value for mussel conservation and extend the identification of associations among mussel species (
Christian et al. 2021) to encompass other species groups. Key examples of the macroinvertebrate taxa identified include the association of Isonychiidae mayflies with the presence of Endangered and Threatened mussel species as well as the specific occurrence of
Ptychobranchus fasciolaris (Kidneyshell) and
Epioblasma triquetra (Snuffbox). Leptohyphidae mayflies were also associated with the presence of Endangered and Threatened mussel species as well as the specific occurrence of
Cyclonaias tuberculata (Purple Wartyback),
Epioblasma rangiana,
Epioblasma triquetra,
Ptychobranchus fasciolaris, and
Pleurobema sintoxia (Round Pigtoe). The ability to identify specific macroinvertebrate species associated with specific Endangered or Threatened mussel species using ITA suggests that macroinvertebrates may have a sufficiently strong relationship to mussels to develop guild or even species-specific indicators that integrate the effects of environmental change and positive species interactions on the most imperiled mussel species. To further develop using macroinvertebrates as an indicator for mussel SAR presence and determine whether associations hold true in reverse (i.e., mussels indicating or predicting macroinvertebrates), continued assessment of the co-occurrence of mussel species and macroinvertebrate taxa across multiple watersheds is encouraged.
Conservation implications
Our results confirm that species co-occurrence matters for conservation and supports the use of multispecies recovery plans involving common species in concert with SAR of extinction. Patterns of species co-occurrences offer critical insight into mechanisms for successful mussel conservation and restoration, as well as potential monitoring approaches. While consideration needs to be given to uniquely adapted species with singular hosts (
Simpsonaias ambigua; Salamander Mussel) or specific soft-sediment habitat requirements (
Toxolasma parvum; Lilliput), our findings demonstrate the need to identify community-level patterns of species co-occurrence to account for the interactions potentially influencing the occurrence and distribution of imperiled species. If facilitation is occurring among mussel species, then recovery plans can go beyond including multiple species in the same plan to actively considering facilitation from more abundant mussel species as a recovery pathway for Endangered and Threatened species. For example, managing for positive interactions among mussel species could involve translocation of mussels as multispecies assemblages (
Mackie et al. 2008), or using existing aggregations of tolerant mussel species as receiving habitats for reintroduction of rare mussels. While these proposed actions may seem a substantial shift in conservation approach and are not without risk, there are clear parallels between using species interactions to restore sedentary mussels and the successful use of facilitation for lizard conservation employing nontrophic interactions with plant species (
Filazzola et al. 2017). Accounting for positive species interactions is common practice for restoring plant communities (
Soliveres et al. 2015) and has underexplored potential among freshwater ecosystems. Within modified, human-settled, and working landscapes such as the Sydenham River watershed complete protection is likely unfeasible or improbable, thus, targeting species interactions through habitat restoration together with population support of multiple species is likely the next best option to facilitate and accelerate SAR recovery. Possible next steps may involve the quantification of mussel–mussel interspecific interactions which can be generalized them across watersheds. If consistent associations between diverse mussel communities and mussel SAR can be established, then monitoring mussel community trends could also serve as a proxy for assessing imperiled mussel species. Within Ontario, monitoring protocols have been demonstrated to be insufficient at tracking small changes in imperiled species populations (
Reid and Morris 2017), thus the incorporation of mussel community diversity and total mussel abundance could allow management targets to be more easily assessed and move recovery of mussels forward.
This study provides evidence that there is reason to re-examine benthic macroinvertebrate records which span broad spatial and temporal scales. Benthic macroinvertebrate diversity is widely utilized both across Ontario (
McGauley et al. 2018) and globally (
Feio et al. 2021), as a bioindicator for aquatic ecosystems due to macroinvertebrates displaying ubiquitous responses to environmental stressors and the relative ease of sampling (
Resh 2008;
Buss et al. 2015). Notably, mussel species richness and macroinvertebrate diversity were highly correlated across the four macroinvertebrate indices analysed. Simple metrics of invertebrate diversity (e.g., macroinvertebrate family richness, HBI scores) and the presence of indicator species are useful for mussel habitat assessment. Within southwestern Ontario, existing data sets collected using standardized protocols (e.g., OBBN) followed here could be used to identify additional hotspots for mussel diversity without additional sampling. Macroinvertebrates are less resource-intensive to sample than mussels, so provide a practical option for screening sites prior to mussel surveys or restoration. The use of macroinvertebrate data adds to existing approaches available for habitat assessment and even offer a preliminary screening tool in areas where local authorities routinely monitor benthic macroinvertebrates. Given that macroinvertebrates respond much more rapidly to environmental change than mussels, trends in macroinvertebrate diversity are likely able to indicate improvements in mussel habitat prior to mussel recovery being observed, although fish monitoring would also be needed to ensure that host fish are present. Integrative indicators of ecosystem state such as macroinvertebrates also hold potential to help harmonize overlapping conservation efforts and communicate the outcomes of management efforts to the public to further promote conservation (
Fitz-Earle and Kobayashi 2008).
Acknowledgements
We gratefully acknowledge funding from the Canada Nature Fund for Aquatic Species at Risk, the NSERC CREATE FishCAST program (to RAE), University of Windsor Graduate Entrance Scholarship (to RAE), Canada Research Chair in Freshwater Restoration Ecology (to CMF), and University of Windsor Start-Up Grant (to CMF). We thank members of the Healthy Headwaters Lab (K. Keeshig, A. Frazao, D. Bresolin, R. Graham, J. Owen, and S. Nolan) for their assistance with fieldwork, sample processing, and organizing logistics. We further thank the St. Clair Region Conservation Authority (SCRCA) for providing advice, monitoring data, and field assistance, as well as landowners in the Sydenham River watershed for allowing site access. Comments from two anonymous reviewers were constructive in refining previous versions of this manuscript.
The survey work was carried out under Species at Risk Act Permit No. 20-PCAA-00024 and no Ontario Ministry of Natural Resources and Forestry or Ministry of the Environment, Conservation and Parks Permit was required due to mussels being immediately released alive. This paper is #180 of the Central Michigan University Institute for Great Lakes Research.