Host traits and lifetime fitness costs of being parasitized in red-breasted mergansers
Abstract
The addition of eggs to a nest by a conspecific is known for approximately 250 bird species. Understanding the evolution of conspecific brood parasitism (CBP) requires assessment of fitness consequences to the egg recipient (host). We addressed host traits and the effects of CBP on future reproduction (i.e., annual survival) and hatching success of hosts by following the nesting of 206 red-breasted mergansers (Mergus serrator) for a colony in which an average of 41% of nests was parasitized annually. Each host was tracked for ≥2 seasons and up to seven seasons. The proportion of a host’s nesting attempts that was parasitized averaged 43% and varied considerably across individuals (range 0%–100%). Probability of parasitism, however, was not repeatable across a host’s nests. Rather, rates of CBP throughout a host’s lifetime increased with earlier dates of nest initiation. CBP had no effect on annual survival of a host. Hatching success throughout a host’s lifetime declined with a greater number of foreign eggs added to the individual’s nests. This study revealed that there may be measurable costs of CBP to lifetime reproductive success in red-breasted mergansers, although our observations suggest that costs to hosts are limited to the most heavily parasitized clutches.
Introduction
The laying of eggs in the nest of an individual of the same species, known as conspecific brood parasitism (CBP), has been observed in approximately 250 bird species (Yom-Tov 2001) and in some insects (Zink 2000), fish (Tariel et al. 2019), and amphibians (Brown et al., 2008). In birds, selection is expected to favour brood parasites that make use of social information to select a suitable nest for parasitism (Pöysä et al. 2014; Eadie and Savard 2015). For example, CBP has been linked to host characteristics that let parasites discriminate among hosts of varying quality (Brown and Brown 1991; 1998). Females that achieve high reproductive success frequently nest early in the breeding season (e.g., Blums and Clark 2004) and, indeed, parasites often target the earliest initiated nests (Clawson et al. 1979; Sorenson 1991; Eadie and Savard 2015). Host experience and body condition may also serve as cues for parasites (Paasivaara et al., 2010; Pöysä et al. 2014; but see Waldeck et al. 2011), because older females in good condition (e.g., with sufficient stored nutrients) may be less likely to abandon their nests than inexperienced hosts in poor condition (Blums et al. 1997).
CBP can be costly to the fitness of the host, who must care for its young and those of the parasite. Among birds that feed their young, broods enlarged through parasitism increase the demand for parental care, which can reduce survival of offspring (Lyon et al. 2002; Tucker et al. 2016) and possibly that of adults (Nur 1984; but see Brown and Brown 1998). In contrast, costs of caring for additional, newly hatched young in species with self-feeding offspring, namely waterfowl, are likely small (Rohwer and Freeman 1989; Dugger and Blums 2001), although there may be costs of CBP during the nesting phase. Waterfowl lay large clutches (Jetz et al. 2008), and incubation of clutches enlarged by parasitism can be inefficient (Caldwell and Cornwell 1975), leading to reduced egg hatchability (Andersson and Eriksson 1982; Semel et al. 1988; Eadie 1989; Sayler 1992; Waltho and Coulson 2015). Large clutches may take longer to incubate (Hepp et al. 1990), forcing females to expend more energy in parental care and prolong exposure to predators (Roy Nielsen et al. 2006a). Greater investment in incubation may ultimately have consequences to annual survival of the host (Andersson 1984), but the idea remains speculative because effects of CBP on host survival have rarely been addressed in waterfowl (but see Rohwer and Heusmann 1991; Milonoff and Paananen 1993).
Assessment of whether CBP affects lifetime reproductive success of the host is required to explore whether brood parasitism is an exploitative relationship with reproductive benefits to the parasite and costs to the host (Eadie et al. 1988; Lyon and Eadie 2008; 2017; Eadie and Savard 2015). In addition, fitness costs of CBP are essential inputs to determine the role of kinship and relatedness in the evolution of brood parasitism (Andersson 1984; McRae and Burke 1996). For example, brood parasites should avoid laying eggs in the nests of kin if CBP is costly to the host (Zink 2000), whereas they are expected to parasitize nests of relatives when costs are low (Andersson 2001; Jaatinen et al. 2011).
This paper explores occurrence and correlates of CBP throughout the lifetime of a host and how the addition of foreign eggs relates to components of her fitness. We focused on a population of red-breasted mergansers (Mergus serrator) in which CBP is an important element of breeding (Young and Titman 1988) and where strong site fidelity has allowed adult females to be followed over their breeding histories (Craik et al. 2020). The study had three main objectives. First, we assessed how lifetime rates of CBP varied across and within hosts by (i) examining the level of repeatability in nest state (parasitized or nonparasitized) over a host’s lifetime and (ii) quantifying the relationship between a host’s current nest state and that during the previous year’s nesting attempt. Second, host traits, namely date of nest initiation, age, and body mass, were assessed as potential cues used by parasites for selecting a host nest. Finally, we explored whether brood parasitism was related to host hatching success and annual survival. We relied on data from a field study in which we monitored nests of 206 red-breasted mergansers, 91 of which were followed over multiple breeding seasons.
Methods
Study site
Nesting by red-breasted mergansers was followed on the Tern Islands at Kouchibouguac National Park, New Brunswick, Canada (46.7769°N, 64.8752°W) during an 18-year period spanning 2002–2019. The Tern Islands is an archipelago of three neighbouring islands that total 3 ha and are 1 km from the mainland. Females construct a nest bowl in the sand within dense stands of marram grass (Ammophila breviligulata). Egg predation on the archipelago is uncommon due in part to high nest concealment (Craik and Titman 2009), the anti-predator mobbing behaviour of co-nesting common terns (Sterna hirundo; Young and Titman 1986), and the absence of mammalian predators. Nests are typically within a 5–20 m distance from water and hens make tunnels within vegetation to facilitate movement between water and nest sites. The small size of the archipelago facilitates the discovery of most, if not all, red-breasted merganser nests in each year. Nesting attempts recorded annually during the study ranged from 31 to 83. Densities of nests (up to 29 nests/ha) are among the highest recorded across the species’ distribution (Craik et al. 2020).
Conspecific brood parasitism is an important feature in the nesting biology of red-breasted mergansers on the archipelago. Craik et al. (2018) assessed CBP at a sample of nests included in the present study and found that on average 48% of nests were parasitized annually, a rate that is relatively high in the range of CBP known from the few studies on this species (Craik et al. 2020). Parasite activity varies seasonally (Young and Titman 1988; Craik and Titman 2009) and, unlike for some other waterfowl, density of nests (Craik and Titman 2009) and aspects of nest-site safety (e.g., concealment; Thimot et al. 2020) are not correlates of CBP at this site (see Pöysä et al. 2014), the latter possibly because rates of nest predation are low. Rather, aspects of host presence are an important, but not necessary, cue used by parasites for selecting a nest to parasitize (Thimot et al. 2020). Both parasitized and unparasitized nests are usually distributed throughout available breeding habitat.
Data collection
We systematically searched nesting habitat for red-breasted merganser nests in June and July of each year. Each nest was marked with an unpainted wooden lath staked 1 m north of the nest bowl. We visited each nest on multiple occasions during laying and incubation to determine nest status and clutch size, and to count eggs outside the nest bowl. For a sample of 38 nests during 2014–2016, we numbered each egg following its addition to the nest to quantify eggs displaced from the nest. No egg losses were observed during the host’s egg-laying cycle, though 39% of incubated clutches lost ≥1 egg for unknown reasons. Parasitized nests are nearly three times as likely to lose an egg for unknown reasons than unparasitized nests, and the number of egg losses at parasitized nests is twice that at unparasitized nests (Craik et al. 2018).
Dates of nest initiation were backdated based on a laying rate of 1 egg each 1.5 day (Craik et al. 2020). For incubated nests, 1 or 2 eggs were floated in a container of water to assess stage of embryonic development (Westerskov 1950). The number of ducklings hatched from a nest was based on counting egg membranes left in the nest bowl. There were a small number of occasions when the number of hatched eggs recorded exceeded the number of membranes because the number of viable eggs observed closely preceding hatching exceeded the number of membranes found (usually a difference of 1 or 2). A difference was justified when (i) a subsequent search revealed a shell and (or) membrane outside but near the nest, or (ii) a nest was examined after a considerable delay since it had hatched, and nest material may have been disturbed by either weather or bird activity at the nest. We calculated hatching success for each successful nest as the proportion of eggs that hatched a duckling.
We assessed parasitism status at nests of the 206 females (n = 370 nests) using three criteria: egg-laying rate, eggs laid after the onset of incubation, and clutch size. These criteria are well-established indicators of conspecific brood parasitism (Eadie et al. 2010; Lyon and Eadie 2017). Only 8% of parasitic eggs are laid after the onset of incubation (Thimot et al. 2020). Egg-laying rate and eggs added during incubation were used to determine occurrence of CBP at 77 of the 370 nests; these nests were found during the earliest stages (median 2 eggs) and visited each 2–3 days through the egg-laying and incubation periods. We assigned the “‘parasitized” status to a nest if eggs were added at a rate exceeding 1 egg each 1.5 day (Craik et al. 2020). Some nest parasitism may have gone undetected prior to nest discovery, and we may have missed CBP if a nest was parasitized during an interval when a host interrupted laying in her own nest to lay parasitically. Analyses of six polymorphic microsatellites from a sample of 10 nests indicated that our field method for detecting CBP was accurate; 9 of 10 nests were classified similarly by genetic and field data (S. Craik, unpublished data). Of the 77 nests, those with ≥12 eggs (n = 28; maximum 22 eggs) were always classified as parasitized based on laying rate and (or) eggs laid during incubation, and 84% (41 of 49) of nests with <12 eggs were not observed to have received a foreign egg. Similarly, Young and Titman (1986) found that clutch size for red-breasted mergansers never surpassed 12 eggs on the North Richibucto Dune, an island located about 1 km from the study site and on which brood parasitism was rarely observed. We thus used clutch size to assign parasitism status to the remaining 293 nests in the study; nests with ≥12 eggs were classified as parasitized and those with <12 eggs were unparasitized. Classifying CBP based on clutch size alone undoubtedly led to an underestimate of CBP given that 16% of parasitized nests with <12 eggs (range 8–11 eggs) were classified incorrectly as nonparasitized nests (see above).
Adult females were captured on the nest during the final week of incubation with a nest trap (Weller 1957). Females were marked with a federal metal leg band at capture, and previously banded individuals were identified. Of the 206 females banded during 2002–2019 (median banded per year: 9, range 5–31), six of these individuals were first captured in the final year of the study and thus did not contribute to survival estimation. Ninety-one females were recaptured at least once, and the mean number of recaptures was 1.8 with a range of 1–6. Captures occurred after all eggs had been laid; thus, a captured bird was most likely the nest’s host. Attempts were made to capture as many hosts as possible each year, but variable weather conditions and logistical constraints meant that capture rates varied annually. Since most, if not all red-breasted merganser nests on the archipelago were discovered each year, we used the proportion of captured females relative to total incubated nests as an annual index of recapture effort (median effort: 62%, ranging from 16%–96%; Supplementary Table S1). We likely missed breeding attempts by some marked females, so estimates for rate of CBP and hatching success throughout the lifetime of these individuals were based on a sample of breeding attempts. There is no reason to believe that failing to capture some incubating females each year would have biased estimates for rates of CBP and hatching success given that hatching success (F1,75 = 0.25, P = 0.62) and probability of CBP (X21 = 1.26, P = 0.26) at incubated nests with a captured host were not different than for incubated nests at which the host was not captured.
We estimated a host’s minimum age based on the number of years since she was first captured. Female red-breasted mergansers do not begin breeding until at least their second year (Craik et al. 2020) and their breeding philopatry is high (Anderson et al. 1992), so we assumed that females captured for the first time were first-time breeders of a minimum age of two and that minimum age at subsequent recaptures was two plus the number of years since first capture. Body mass was measured using a Pesola scale (±5 g). Ideally, host body mass is measured when most parasites are selecting host nests (i.e., during egg laying); however, trapping hosts during egg laying increases chances for nest desertion.
All procedures involving animals were in accordance with (i) the Canadian Council on Animal Care (CCAC) guidelines and (ii) the ethical standards of the institutions that led fieldwork (Université Sainte-Anne animal use protocol #06–19 and McGill University animal use protocols #1926 and #7329).
Statistical analyses
Occurrence of CBP and host traits
We used repeatability analyses to examine whether there were stable individual differences in nest state (parasitized or nonparasitized) over a host’s lifetime (Nakagawa and Schielzeth 2010). Repeatability (R) describes the proportion of variance in a trait that occurs among rather than within individuals, and is calculated as the variance among individuals (group-level variance; VG) over the sum of group-level and within-group (residual) variance VR:
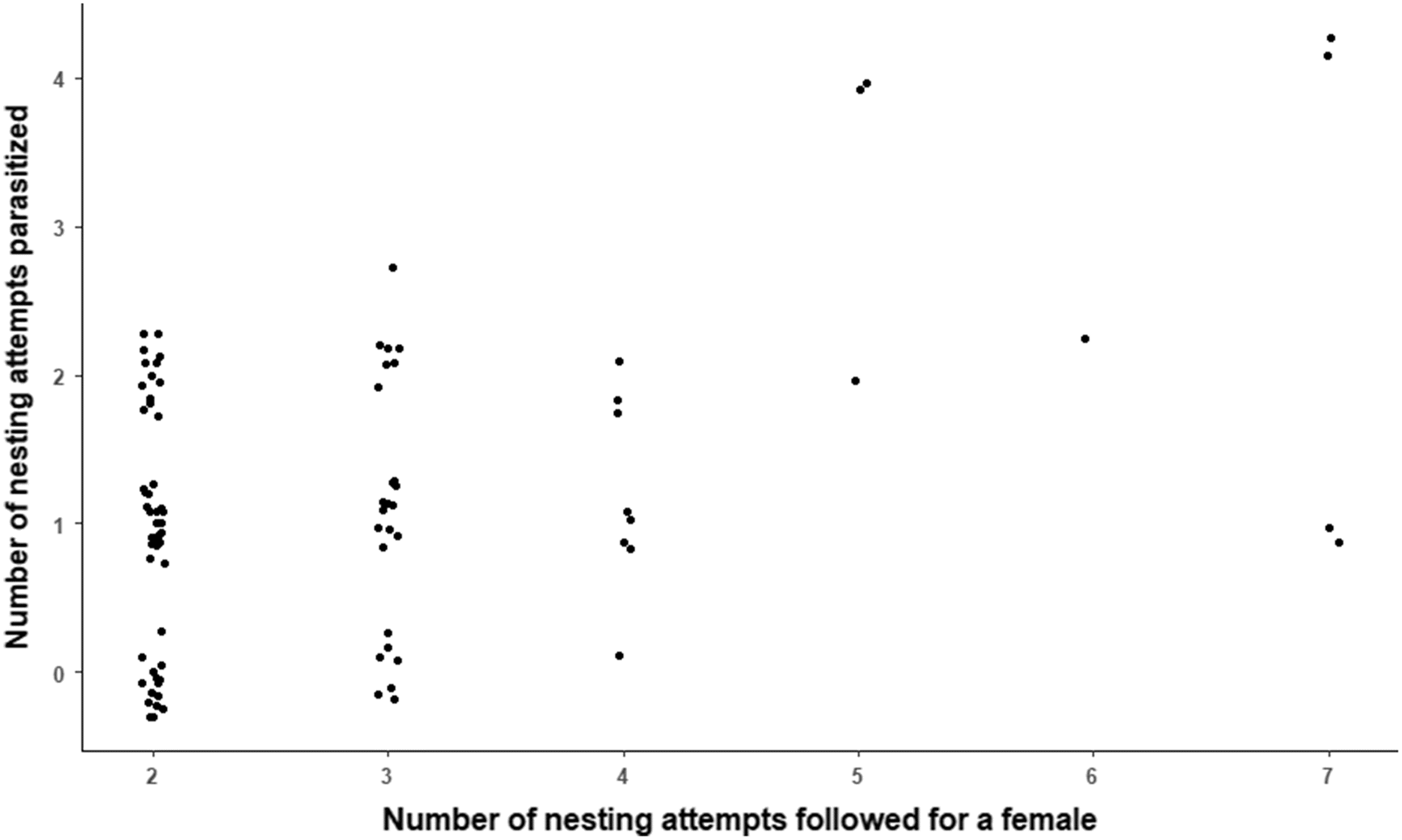
We assessed repeatability in nest state for the 91 individuals captured during ≥2 breeding seasons (mean 3 seasons, range 2–7) using the generalized linear mixed-model method with logit link in package rptr (Stoffel et al. 2017) for R 4.0.3 (R Core Team 2020). We coded nest state as a binary variable (0 = nonparasitized; 1 = parasitized) and female identity as a random factor. Repeatability was estimated on the latent scale and uncertainty in estimates was assessed by calculating 95% bootstrap confidence intervals (1000 bootstraps; Nakagawa and Schielzeth 2010). Statistical significance against the null hypothesis of no repeatability (R = 0) was tested by comparing observed repeatability to a distribution generated from 1000 randomizations (Stoffel et al. 2017). We used the term “number of nesting attempts over a lifetime” rather than “number of seasons over a lifetime” because some females may renest in a season (Craik et al. 2020). The number of nesting attempts for which a female incubated a parasitized clutch increased with the number of nesting attempts followed (F1,87 = 20.8; P < 0.001), so we conducted separate repeatability analyses for females followed on two and three occasions to control for number of nesting attempts monitored. Separate analyses were not performed for females followed over ≥3 nesting attempts because sample sizes were small (Fig. 1).
Fig. 1.
We used generalized linear mixed models (GLMM) with logit link in R 4.0.3 (R Core Team 2020) to assess the role of host traits on probability of CBP (lme4 package; Bates et al. 2015). Eleven nesting attempts were censored from analysis because host body mass (n = 9) or date of nest initiation (n = 2) were unknown. Nest state was a binary response variable (0 = nonparasitized; 1 = parasitized), host traits (date of nest initiation, body mass, minimum age) and year were fixed effects, and female identity was a random effect. Nest initiation dates in each year were standardized (i.e., day 1 = first day an egg was laid) to account for annual variation in nesting schedule. We examined the level of association among fixed effects by computing Pearson’s product-moment correlation coefficients. Host body mass and minimum age did not vary with date of nest initiation (t239 = −0.59, P = 0.56; t239 = 0.62, P = 0.54, respectively). Body mass and minimum age were positively correlated (t239 = 2.15, P = 0.03).
A set of candidate models was established by first fitting a global GLMM that included all fixed and random effects. Next, we generated a set of 16 models from the global model that included all combinations of single and additive fixed effects, and an intercept-only model with random effects (Grueber et al. 2011). An information-theoretic approach based on Akaike’s Information Criterion corrected for small sample size (AICc) was used to assess the importance of models and their parameters (Akaike 1973). Selection of the best approximating model(s) was based on the values of ΔAICc, calculated as the difference in values of AICc between the model of interest and the model with the lowest AICc value (Burnham and Anderson 2002). We calculated the weight (wi) of each model because models with larger weights better approximate the data. The potential for each fixed effect to discriminate between parasitized and unparasitized nests was assessed in two ways. First, weights of all models with a particular fixed effect were summed to assess the predictor’s relative importance; the larger the sum of weights, the more important the variable is relative to others (Burnham and Anderson 2002). Second, we model-averaged slopes and generated unconditional 95% confidence intervals at the scale of the linear predictor from the best approximating model(s) (ΔAICc < 2; Burnham and Anderson 2002). We concluded lack of fit when effect size did not differ from zero (i.e., confidence intervals included zero). The model selection exercise was then repeated twice, using (i) only those nests assessed for CBP with egg-laying rate and eggs laid after onset of incubation, and (ii) only those nests assessed for CBP based on the clutch size criterion. Results of these two analyses were similar to those obtained using the entire data set (see Supplementary Tables S2 and S3), so we only present results from the entire data set below.
CBP and host fitness
Hatching success
We were interested in the relationship between CBP and hatching success, so nests abandoned following host capture (n = 35) were censored from analyses. Overall, rate of nest abandonment at parasitized nests (10.8% of 102 nests) was similar to nonparasitized nests (16.7% of 144 nests; X21 = 1.25, P = 0.26), and abandoned nests were no different than successful nests with respect to date of nest initiation (F1,234 = 0.11, P = 0.74), minimum age of the host (F1,234 = 0.38, P = 0.54), and host body mass (F1,234 = 2.45, P = 0.12).
The source of each egg in a parasitized nest was unknown, and we assumed that hatching success of host and parasite eggs in a clutch was similar. Hosts suffer from lowered hatching success when parasites reject host eggs (Lombardo et al. 1989), but host egg rejection by parasites seems unlikely in red-breasted mergansers given that egg loss was not observed during the host’s egg-laying period (see above). In waterfowl, parasite eggs suffer lower hatchability than host eggs when they are added after the onset of incubation (Eadie 1989; Sorenson 1991; Roy Nielsen et al. 2006a); however, brood parasites in red-breasted mergansers rarely add eggs to an incubated nest (Thimot et al. 2020).
Factors linked to hatching success were examined with GLMMs. Hatching success in red-breasted mergansers declines with clutch size (Craik et al. 2018), so we explored the effects of the number of parasite eggs in a clutch on hatching success. The number of parasite eggs in a nest was estimated as the number of eggs exceeding 11, because nearly 80% of all parasitized nests had ≥12 eggs (see above). This method likely underestimated magnitude of CBP at some nests and missed brood parasitism at several nests with <12 eggs. The response variable was hatching success; fixed effects were number of parasite eggs, year, and host date of nest initiation, minimum age and body mass; and female identity was a random effect. We generated a set of 32 models from the global model that contained all combinations of single and additive fixed effects and an intercept-only model containing random effects. Selection of the best approximating model(s) and assessment of the relative importance of each fixed effect were performed as described above.
Hatching success over a host’s lifetime was estimated as the proportion of all eggs that hatched from her nesting attempts. The total number of parasite eggs added to a hen’s nests did not vary with number of nesting attempts followed (ANOVA: F3,68 = 2.46, P = 0.07). We thus assessed costs of CBP by exploring the relationship between hatching success over a lifetime and the total number of parasite eggs added to the individual’s nests using logistic regression.
Annual survival and nest-state transition probabilities
Our main objectives were to (i) estimate annual survival probability of hosts, (ii) determine whether hosts incubating a parasitized clutch have reduced annual survival rates, and (iii) determine how the probability of parasitism varies over time and with previous parasitism (Burnham et al.et al. 1992). We employed multi-state mark-recapture models through Program MARK (White and Burnham 1999) using the RMark (Laake 2013; 2014) package in R 4.0.3 (R Core Team 2020) allowing simultaneous estimation of apparent annual survival (S) and recapture (p) probabilities for each of the two nest states (parasitized nest and nonparasitized) and probability of transition (ψ) between the two states. Multi-state encounter histories encoding nest state at initial captures (n = 206 birds) and recaptures were generated using RMark. Date of nest initiation and body mass were only known at recapture occasions and incorporating mean values as individual covariates would not be meaningful. Further, the data were too sparse to include time-varying dates of nest initiation and body mass, and since these covariates cannot include missing values for capture occasions where individuals are not encountered, we deemed it unfeasible to include date of nest initiation and body mass as covariates in our models.
We tested the fit of the most general multi-state model {Sstate*time pstate*time ψstate*time} to the data using Arnason–Schwartz multi-state goodness-of-fit procedures (package R2ucare, Gimenez et al. 2018) to identify any biologically relevant sources of poor model fit (e.g., transience, trap-dependence; Pradel et al. 2003). We also used bootstrap and median-ĉ simulations (100 replicates in each case) on a single-state (Cormack–Jolly–Seber) model structure in Program MARK to quantify any overdispersion in the data requiring adjustment of the deviance values when calculating AIC (Akaike 1973) for model selection. Models were compared via differences in AICc values and relative model weights (Burnham and Anderson 2002), and support for specific predictors was evaluated from effect sizes evaluated at the scale (logit) of the linear predictor.
The fully saturated general model {Sstate*time pstate*timeψstate*time} had close to 100 parameters, and many of the parameters were poorly estimated. To reduce the dimensionality of the parameter space, we explored reduced parameter models of p and saw evidence that the effort-index of capture rate improved model fit over state- or time-variant models based on AICc and effect sizes. We also explored models that allowed for differing survival rates in the interval after first capture relative to subsequent years (age or transiency effects, Pradel et al. 2003), but found no evidence that this effect was present in the data set (TEST 3.SR not significant); we thus did not consider minimum age in our candidate model set.
Our starting model included interactive time and state variation for S and ψ, and p was constrained to co-vary with capture effort {Sstate*time peffort ψstate*time}. The candidate model set included combinations of these three parameters under further constraints. Survival could vary among years (Stime), between the two nest states (Sstate), or not at all (S). Transition probabilities could vary among years (ψtime), with nest state (ψstate, where probability of changing from parasitized-to-non among consecutive nesting attempts differs from that of non-to-parasitized) or not at all (ψ.). To ensure that our inferences around S and ψ were robust to the constraint of capture effort we placed on p, we refit the top models without the effort constraint on p (i.e., ptime), and found that relative ranking among these models was largely similar to the effort-models but that standard error on parameter estimates were much wider, as expected.
Results
Occurrence of CBP and host traits
The annual proportion of all nests on the archipelago that were parasitized by conspecifics averaged 41% and ranged from 19% to 53% (Supplementary Table S1). On average, 3.6 ± SE 0.3 (range 1–11) foreign eggs were estimated to have been added to a parasitized nest. For a given host, the mean number of nesting attempts that were parasitized was 1.2 ± SE 0.1 (Fig. 1), and the proportion of her nesting attempts that were parasitized averaged 43% ± SE 4% and ranged from 0% to 100%. Still, we failed to find stable individual differences in rates of CBP across a host’s lifetime when considering the entire data set (R = 0.04, 95% CI: 0, 0.15; P = 0.14) or only those females followed over two (R = 0.19, 95% CI: 0, 0.34; P = 0.10) or three nesting attempts (R = 0, 95% CI: 0, 0.23; P = 0.57).
Standardized date of nest initiation at parasitized nests (10.5 ± SE 0.7) was on average a week earlier than nonparasitized nests (17.7 ± SE 0.7; F1,239 = 45.6; P < 0.001; Fig. 2A). Nests initiated late in the season rarely received foreign eggs (Supplementary Fig. S1). Host body mass and minimum age were not correlates of CBP (Fig. 2B–C). Three lines of evidence from our model selection exercise support these observations. First, each of the four models that best discriminated parasitized and unparasitized nests (ΔAICc < 2) included date of nest initiation (Table 1). Second, the sum of model weights for date of nest initiation was a maximum value (1.0) and at least twice as great than host body mass (0.48) and minimum age (0.29) and year (0.05). Finally, date of nest initiation was the only fixed effect for which an unconditional estimate of the 95% confidence interval of the model-averaged slope did not include zero, confirming that probability of CBP declined with later dates of nest initiation (β = −0.98, 95% CI: −1.33, −0.63).
Fig. 2.

Table 1.
| Model | Deviance | Parameters | ΔAICc | wi |
|---|---|---|---|---|
| Date of nest initiation + body mass | 281.342 | 4 | 0.000 | 0.343 |
| Date of nest initiation | 283.485 | 3 | 0.074 | 0.330 |
| Date of nest initiation + minimum age | 283.089 | 4 | 1.747 | 0.143 |
| Date of nest initiation + body mass + minimum age | 281.141 | 5 | 1.884 | 0.134 |
| Date of nest initiation + year | 251.213 | 20 | 5.520 | 0.022 |
| Date of nest initiation + body mass + year | 249.697 | 21 | 6.405 | 0.014 |
| Date of nest initiation + minimum age + year | 250.548 | 21 | 7.255 | 0.009 |
| Date of nest initiation + body mass + minimum age + year | 249.135 | 22 | 8.265 | 0.005 |
Note
Each model is shown with corresponding deviance, number of parameters, difference in AICc value from the best-fitting model (ΔAICc), and relative ΔAICc weights (wi). All models include an intercept and female identity as a random factor. The minimum AICc value (i.e., for the best-fitting model) was 289.512. Data are from 241 nests.
To better understand factors contributing to seasonality in CBP, we examined for individual differences in dates of nest initiation across a lifetime using a repeatability analysis (see Methods for description of analysis). We detected some repeatability in dates of nest initiation, although the effect size was marginal (R = 0.12, 95% CI: 0, 0.26; P = 0.03). The proportion of a female’s nesting attempts that was parasitized decreased with later mean dates of nest initiation across her lifetime (likelihood-ratio test: X21 = 26.3, P < 0.001; Fig. 3). Fourteen females that were parasitized during each of their nesting attempts had some of the earliest mean dates of nest initiation, whereas 23 hens that were never parasitized tended to nest later each year (Fig. 3). Still, some females nested early during some years and later during others, and their lifetime rates of CBP reflected this variability in nesting schedule (i.e., some early nests parasitized and later nests rarely parasitized; see Supplementary Fig. S2 for several examples).
Fig. 3.

CBP and host fitness
Hatching success
Mean hatching success at parasitized nests (0.59 ± SE 0.02; n = 124 nests) was 11% lower than at nonparasitized nests (0.70 ± SE 0.02; n = 177 nests). This observation reflected a decline in hatching success with greater clutch size (likelihood ratio test: X21 = 69.9, p ≤ 0.001). Indeed, hatching success was lowest for clutches with ≥14 eggs (Fig. 4), which represented 22% of all nests in the study. Hatching success declined with a greater number of parasite eggs added to the nest (model-averaged β = −0.31, 95% CI: −0.42, −0.20). Similarly, hatching success over a lifetime declined with a greater total number of parasite eggs added to the hen’s nests (likelihood ratio test: X21 = 13.9, P ≤ 0.001; Fig. 5). Number of parasite eggs was included in each of the five models that best approximated variation in hatching success and the sum of weights for models including number of parasite eggs was 1 (Table 2). Each of the top models also included year effects (Table 2), at least in part because hatching success was generally lowest in years with the highest rates of CBP (likelihood-ratio test: X21 = 48.2, P < 0.001). Host nest initiation date (model-averaged β = −0.08, 95% CI: −0.13, 0.08), minimum age (β = 0.11, 95% CI: −0.08, 0.21), and body mass (β = 0.14, 95% CI: −0.04, 0.28) did not predict hatching success.
Fig. 4.

Fig. 5.

Table 2.
| Model | Deviance | Parameters | ΔAICc | wi |
|---|---|---|---|---|
| No. parasite eggs + body mass + minimum age + year | 487.933 | 22 | 0.000 | 0.248 |
| No. parasite eggs + body mass + year | 490.764 | 21 | 0.314 | 0.212 |
| No. parasite eggs + minimum age + year | 491.845 | 21 | 1.394 | 0.124 |
| No. parasite eggs + date of nest initiation + body mass + minimum age + year | 486.831 | 23 | 1.443 | 0.121 |
| No. parasite eggs + date of nest initiation + body mass + year | 489.643 | 22 | 1.710 | 0.106 |
| No. parasite eggs + year | 495.277 | 20 | 2.337 | 0.077 |
Note
Each model is shown with corresponding deviance, number of parameters, difference in AICc value from the best-fitting model (ΔAICc), and relative ΔAICc weights (wi). All models include an intercept and female identity as a random factor. The minimum AICc value (i.e., for the best-fitting model) was 537.555. Data are from 203 nests.
Annual survival and nest-state transition probabilities
The goodness-of-fit tests consistently indicated that there was no overdispersion in our data. Tests for transience of individuals (Test 3.GSR) and trap-dependence (Test M.ITEC) were nonsignificant. Bootstrap, median-ĉ, and Fletcher (Fletcher 2012) estimates all suggested a ĉ value below 1. As a result, we made no adjustment (i.e., we kept ĉ = 1), and proceeded to evaluate our models based on AICc.
Several models received similar support from our candidate model set (Table 3). Time-variation was not supported for either survival or transition probability. For survival, there was similar support between the model with no variation {S peffort ψ} and with differences between nest states {Sstate peffort ψ} (ΔAICc < 2). However, the state-varying effect size did not differ from zero (logit-link βSstate = 0.129, 95% CI: −0.543, 0.802), indicating that probability of annual survival is similar between females with parasitized and nonparasitized clutches. There was similarly marginal support for state differences in transition probability, with similar rankings between models with {S peffort ψstate} and without {S peffort ψ} this variation (ΔAIC < 2, logit-link βψparasitized-to-non = −0.003, 95% CI: −0.548, 0.541; βψnon-to-parasitized = −0.333, 95% CI: −0.803, 0.137).
Table 3.
| Model | Deviance | Parameters | ΔAICc | wi |
|---|---|---|---|---|
| S peffort ψ | 578.261 | 4 | 0 | 0.442 |
| S peffort ψstate | 577.117 | 5 | 0.913 | 0.280 |
| Sstate peffort ψ | 578.119 | 5 | 1.916 | 0.170 |
| Sstate peffort ψstate | 576.970 | 6 | 2.837 | 0.107 |
| Stime peffort ψ | 559.227 | 20 | 15.387 | 0.000 |
| S peffort ψtime | 559.347 | 20 | 15.508 | 0.000 |
| Stime peffort ψstate | 558.082 | 21 | 16.505 | 0.000 |
| Sstate peffort ψtime | 558.591 | 21 | 17.015 | 0.000 |
| Sstate*time peffort ψ | 532.597 | 37 | 29.176 | 0.000 |
| Sstate*time peffort ψstate | 530.666 | 38 | 29.759 | 0.000 |
| S p ψ | 614.440 | 3 | 34.132 | 0.000 |
| Sstate*time peffort ψstate*time | 487.615 | 70 | 76.612 | 0.000 |
Note
Each model is shown with corresponding deviance, number of parameters, difference in AICc value from the best-fitting model (ΔAICc), and relative ΔAICc weights (wi). Model parameters include probabilities of survival (S), recapture (p) and transition between parasitized and non-parasitized states (ψ). Subscripts indicate variation among years (time), between states (state), with capture effort (effort), and interactive combinations of these (*). The minimum AICc value (i.e., for the best-fitting model) was 1029.771. Data are from 206 individuals.
The model in which survival and transition are time and state invariant and recapture probability varied with effort {S peffort ψ} appeared to be the best fit for our data (Table 3). The model gave a mean annual survival estimate of S = 0.684 (SE 0.023, 95% CI: 0.637, 0.728). Mean annual recapture probability p varied from 0.109–0.689 as a function of the recapture effort index. State-transition probabilities under this model were equal between the two states, such that the probability of changing states between nesting attempts was ψparasitized-to-non = ψnon-to-parasitized = 0.451 (SE 0.051, 95% CI: 0.354, 0.552). The probability of not changing states between nesting attempts was therefore the complement of this, i.e., ψparasitized-to-parasitized = ψnon-parasitized-to-non-parasitized = 0.549 (SE 0.051, 95% CI: 0.448, 0.646). A host’s nest was therefore just as likely to change CBP states over consecutive nesting attempts as it was to remain the same.
In light of these findings, we present an apparent survival rate calculated with a variance components approach (function var.covar within RMark) via a time-varying survival single-state Cormack–Jolly–Seber model structure {φtime peffort} for which the amount of time-dependent process variation (σ2) in survival probabilities can be determined. For comparison with other studies or use for management purposes, our mean estimate of apparent survival considering sampling error was 0.657 ± SE 0.025, with a process variance (σ2 = 0.0005, 95% CI: −0.005–0.03) accounting for only a small proportion of the total annual variance (process + sampling variance) in the data.
Discussion
We followed nesting by a host for an average of three seasons and up to seven seasons. A couple of lines of evidence suggest that at least some females were followed for at least most of their reproductive years. First, the top-ranked mark-recapture model gave an annual survival estimate of 68%, implying that females are unlikely to live beyond 7–8 years. Indeed, only 2% of females banded on the archipelago since 2002 nested beyond their eighth year. Since female red-breasted mergansers do not begin breeding until at least their second year, the reproductive life for many hosts likely does not surpass four or five seasons. Second, capture histories for several individuals revealed an interval of time during which she was followed in most years (nesting gaps usually of ≤2 years) after which she was never observed again. Goodness-of-fit tests showed no evidence of short-term transience from the archipelago, indicating strong philopatry to the breeding site (Anderson et al. 1992). Absence of a female during multiple years following a time interval when she nested suggests the individual had died.
Rates of CBP over a host’s lifetime were strongly affected by dates of nest initiation because parasite activity declines throughout the season. High rates of CBP early in the season are consistent with previous reports from the study site (Young and Titman 1988; Thimot et al. 2020) and for a number of other waterfowl (Clawson et al. 1979; Sorenson 1991; Robertson et al. 1992; Paasivaara et al. 2010; Eadie and Savard 2015). Some of the variability in lifetime rates of CBP reflected individual repeatability in dates of nest initiation. For example, females that typically nested early in the season had some of the highest rates of CBP at their nests, whereas hosts that were never or rarely parasitized often nested later in the season. Timing of breeding in waterfowl has been linked to host condition and experience (e.g., Blums and Clark 2004); however, date of nest initiation in red-breasted mergansers was unaffected by host body mass during incubation or by the host’s minimum age. An important unresolved question, then, is what drives timing of breeding in red-breasted mergansers? Body condition before breeding (Devries et al. 2008) and timing of pair formation (Spurr and Milne 1976) are correlates of nesting schedule in some birds, so their consideration in the red-breasted merganser may reveal important insight to factors contributing to lifetime rates of CBP for hosts in this species.
Seasonality in CBP for red-breasted mergansers may reflect adaptive behaviour by brood parasites. By parasitizing early on, females may have sufficient time to complete their own nest prior to the end of the season (Gibbons 1986; Lyon 1993; Åhlund and Andersson 2001; Reichart et al. 2010), a tactic that can favour high reproductive success relative to nesting-only or parasite-only strategies (Sorenson 1991; McRae 1998; Åhlund and Andersson 2001; Lyon and Eadie 2008). Given the strong tendency for red-breasted mergansers to parasitize nests early in the season, it may very well be that some females employ the dual tactic of parasitism plus nesting within the same season.
It seems unlikely that most brood parasites select nests of specific hosts to lay foreign eggs (i.e., host identity is not an important cue for parasites). Consistent with this idea were observations that (i) there were no stable individual differences in occurrence of CBP across a host’s lifetime and (ii) that a host’s nest was just as likely to change CBP states in consecutive nesting attempts as it was to remain in the same state. Host behaviour may limit opportunities for brood parasites to obtain information on the identity of the host. During egg laying (i.e., when parasite activity is greatest), red-breasted mergansers spend ∼90% of the day away from the nest and only visit it briefly to lay an egg each 1–2 days (Noel et al. 2021), so there are likely opportunities for prospecting brood parasites to visit a nest at which the host is absent. Alternatively, brood parasites may obtain information on the identity of a nest’s host by following the host to its nest, as has been observed for some cavity-nesting waterfowl (Heusmann et al. 1980; Pöysä et al. 2014). Still, red-breasted mergansers parasitize experimental nests without a host (Thimot et al. 2020) and lay in abandoned nests (Craik and Titman 2009), confirming that host identification is not a requisite for parasitic egg laying in this bird (Eadie 1989; Pöysä 2003; Odell and Eadie 2010).
There is little evidence suggesting that red-breasted mergansers try to avoid having their nest parasitized by conspecifics. Hosts can reduce the ease by which parasites find their nests by selecting sites that are isolated or less visible to conspecifics (Roy Nielsen et al. 2006b; Pöysä et al. 2014). However, parasitized and nonparasitized nests of red-breasted mergansers on the archipelago are surrounded by similar densities of conspecific nests (Craik and Titman 2009) and have equally high levels of vegetative concealment (Thimot et al. 2020). Hosts in some birds resist CBP via rejection of parasite eggs (Emlen and Wrege 1986; Lyon 1993; McRae 1995; 2011; Jamieson et al. 2000) or physically blocking a parasite’s entry to the nest (Clawson et al. 1979; Emlen and Wrege 1986; McRae 1996; Sorenson 1997; Andersson et al. 2015). Red-breasted mergansers seem no different than other ducks in that they do not remove parasite eggs from their nest immediately after being laid (Sayler 1992), and opportunities for hosts to resist brood parasites are limited given that hosts spend little time at the nest when parasite activity is greatest (Noel et al. 2021). The lack of strong host defense against CBP in this species may reflect constraints due to a long egg-laying period (e.g., up to 16–17 days; Craik et al. 2020) or lack of selective pressure given that fitness costs of CBP may be small for many hosts (see below).
Hatching success at parasitized nests was 11% lower than at nonparasitized nests. Egg hatchability at parasitized nests on the archipelago may be lowered by (i) embryo mortality due to inefficient incubation of large clutches (Young and Titman 1988) or (ii) egg displacement during incubation (Craik et al. 2018). We caution, however, that effects of CBP on hatching success were overestimated because the clutch-size criterion missed brood parasitism in nests with ≤11 eggs, and hatching success in these nests is relatively high. Craik et al. (2018) relied on egg-laying rates only to assess CBP from a sample of nests in this study, and they reported no difference in hatching success between parasitized and nonparasitized nests. Nonetheless, this does not take away from the fact that hatching success in the most heavily parasitized nests (≥14 eggs) that produce young was lower relative to parasitized nests with smaller clutches and nonparasitized nests. Hatching success in a nest declined with a larger number of foreign eggs, and hatching success over the lifetime of a host declined with a greater total number of parasitic eggs added to the individual’s nests. Together, these results suggest that there are measurable costs of CBP to host hatching success, though they are generally limited to the proportion of hens incubating the most heavily parasitized nests each year (20%–25% of all nests). Effects of CBP on hatching success for hens incubating smaller clutches appear to be negligible (Craik et al. 2018).
We missed data from nests deserted during egg laying and early incubation because we relied on a sample of females identified during late incubation. Indeed, any year within an individual’s breeding history during which she was not captured may have reflected a failed breeding attempt. Nest desertion is common in this population (Young and Titman 1986; Craik and Titman 2009). On average, 18% of nests were abandoned during the egg-laying phase, and hens occasionally abandon nests during incubation (e.g., 18% of 106 incubated nests were deserted; Craik and Titman 2009). A high magnitude of CBP triggers nest abandonment in some waterfowl (e.g., Andersson and Eriksson 1982; Eadie 1989), and this may be the case in red-breasted mergansers. Craik et al. (2018) found that nests receiving 2–6 experimental eggs over three days were occasionally abandoned, whereas control nests without parasitism were always incubated. Desertion of a heavily parasitized clutch may represent an important cost to fitness of a host, particularly if she does not lay the rest of her eggs elsewhere.
Annual survival probabilities for hosts tending a parasitized nest were no different than for females with a nonparasitized nest. Parents that care for large clutches may need to prolong incubation to bring eggs to term (Hepp et al. 1990; but see Milonoff and Paananen 1993), which extends energetic investment in parental care (Roy Nielsen et al. 2006a) and lengthens the amount of time hosts are exposed to nest predators. Craik et al. (2018) found no difference in incubation period between parasitized and unparasitized nests on the archipelago, while in an earlier study Young and Titman (1986) indicated that parasitism may extend incubation by about one day in some nests, although they did not provide a statistical test. Regardless, a slightly greater incubation period is unlikely to increase risk of female predation at the nest because mammalian predators are absent from these islands and co-nesting common terns are efficient at driving avian predators away from the colony (Young and Titman 1986).
We caution that our conclusion that broods enlarged by CBP do not affect annual survival of hosts should not extend beyond the nesting phase. The number of ducklings leaving a nest is not a reliable indicator of initial brood size for red-breasted mergansers because amalgamation of broods can occur shortly following departure from the nest (Craik et al. 2020). Brood size may affect survival of hosts that feed their young (Nur 1984) but may not be the case in birds wherein young are not fed (Rohwer and Freeman 1989). Empirical data, although limited to a small number of studies with ducks (Rohwer and Heusmann 1991) and geese (Lessels 1986; Lank et al. 1990; Larsson et al. 1995) support the premise that female annual survival in waterfowl is unaffected by brood size. Unlike geese, ducks do not extend parental care beyond fledging, so an association between brood size and annual survival of brood-rearing females is unexpected in the red-breasted merganser.
Results of the multi-state mark-recapture modelling filled a significant gap in our knowledge of sea duck (Mergini) life history by providing the first estimate of apparent adult survival for the red-breasted merganser, at 0.68 per year, very close to expectations based on an average unparasitized clutch size just over 9 eggs (see fig. 10.6 in Mallory 2015). This value places red-breasted merganser annual survival similar to that of other sea ducks like goldeneyes and buffleheads (Bucephala spp.; 60%–67%), and in the broad but sparsely studied range of other mergansers (49%–72%), and lower than apparent survival in scoters and eiders (>75%; see review in Mallory 2015). Principal causes of mortality for adult red-breasted mergansers are largely unknown (Craik et al. 2020).
High-density nesting in waterfowl can lead to frequent CBP (Sayler 1992), which in turn may lead to fitness costs for hosts (Eadie and Savard 2015). Nest densities and rates of CBP for red-breasted mergansers on the archipelago are among the highest known for this species (Craik et al. 2020) and are high for birds in general (Sayler 1992; Eadie and Savard 2015; Lyon and Eadie 2017). Despite its common occurrence and often high magnitude at a nest, CBP had no effect on future reproduction (i.e., annual survival), and measurable costs of brood parasitism to hatching success were generally limited to the most heavily parasitized clutches, which represented ∼20%–25% nests annually. We emphasize, however, that some nest abandonment is likely triggered by heavy CBP, implying that our study may have overlooked other costs of CBP to hosts with heavily parasitized nests.
Acknowledgements
We are grateful to D. Bilodeau, A. Constantineau, M. Deveau, K. Francis, J. Haché, A. Hanks, N. Laplante, F. Leblanc, M. Mangoni, A. Rousseau, K.S. Seaborn, B. Spinney, N. Thimot, and the Titman family for their assistance in the field. Kouchibouguac National Park staff, particularly A. Beaudet, D. Gallant, the late P-É Hébert, B. Martin, and É. Tremblay provided invaluable logistical support. J.M. Eadie, B.E. Lyon, and four anonymous referees provided very helpful comments on earlier versions of the manuscript. This work was supported by the Natural Sciences and Engineering Research Council of Canada (DDG-2019-06038 to S.R.C.), New Brunswick Wildlife Trust Fund, McGill University, Bird Protection Québec, Sea Duck Joint Venture, and Université Sainte-Anne.
References
Åhlund M, and Andersson M. 2001. Female ducks can double their reproduction. Nature, 414: 600–601.
Akaike H. 1973. Information theory as an extension of the maximum likelihood principle. In Second international symposium on information theory. Edited by BN Petrov and F Csaki.Akademiai Kiado, Budapest, Hungary. pp. 267–281.
Anderson MG, Rhymer JM, and Rohwer FC. 1992. Philopatry, dispersal, and the genetic structure of waterfowl populations. In Ecology and management of breeding waterfowl. Edited by Batt BDJ, Afton AD, Anderson MG, Ankney CD, Johnson DH, Kadlec JA, and Krapu GL. University of Minnesota Press, Minneapolis, MN. pp. 365–395.
Andersson M. 1984. Brood parasitism within species. In Producers and scroungers: strategies of exploitation and parasitism. Edited by Barnard CJ. Croom Helm, London, UK. pp. 195–228.
Andersson M. 2001. Relatedness and the evolution of conspecific brood parasitism. The American Naturalist, 158: 599–614.
Andersson M, and Eriksson MOG. 1982. Nest parasitism in goldeneyes Bucephala clangula: Some evolutionary aspects. The American Naturalist, 120: 1–16.
Andersson M, Waldeck P, Hanssen SA, and Moe B. 2015. Female sociality and kin discrimination in brood parasitism: Unrelated females fight over egg laying. Behavioral Ecology 26: 755–762.
Bates D, Maechler M, Bolker B, and Walker S. 2015. lme4: Linear Mixed-Effects Models Using Eigen and S4. R package version 1.1–10. [online]: Available from CRAN.R-project.org/package=lme4.
Blums P, and Clark RG. 2004. Correlates of lifetime reproductive success in three species of European ducks. Oecologia, 140: 61–67.
Blums P, Mednis A, and Clark RG. 1997. Effect of incubation body mass on reproductive success and survival of two European diving ducks: a test of the nutrient limitation hypothesis. Condor, 99: 916–925.
Brown CR, and Brown MB. 1991. Selection of high-quality host nests by parasitic cliff swallows. Animal Behaviour, 41: 457–465.
Brown CR, and Brown MB. 1998. Fitness components associated with alternative reproductive tactics in cliff swallows. Behavioral Ecology, 9: 158–171.
Brown JL, Morales V, and Summers K. 2008. Tactical reproductive parasitism via larval cannibalism in Peruvian poison frogs. Biology Letters, 5: 148–151.
Burnham KP, and Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. 2nd edition. Springer, Berlin, Germany.
Burnham KP, Clobert J, and Anderson DR. 1992. Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecological Monographs, 62: 67–118.
Caldwell PJ, and Cornwell GW. 1975. Incubation behavior and temperatures of the Mallard duck. Auk, 92: 706–731.
Clawson EL, Hartman GW, and Fredrickson LH. 1979. Dump nesting in a Missouri wood duck population. Journal of Wildlife Management, 43: 347–355.
Craik S, Pearce J, and Titman RD. 2020. Red-breasted Merganser (Mergus serrator), version 1.0. In Birds of the world. Edited by SM Billerman. Cornell Lab of Ornithology, Ithaca, NY, USA.
Craik SR, and Titman RD. 2009. Nesting ecology of red-breasted mergansers in a common tern colony in eastern New Brunswick. Waterbirds, 32: 282–292.
Craik SR, Titman RD, Savard JPL, Kaouass M, Thimot N, Elliott KH, and Tremblay É. 2018. Costs and response to conspecific brood parasitism by colonial red-breasted mergansers. Journal of Ethology, 36: 251–258.
Devries JH, Brook RW, Howerter DW, and Anderson MG. 2008. Effects of spring body condition and age on reproduction in mallards (Anas platyrhynchos). Auk, 125: 618–628.
Dugger BD, and Blums P. 2001. Effect of conspecific brood parasitism on host fitness for tufted duck and common pochard. Auk, 118: 717–726.
Eadie JM. 1989. Alternative reproductive tactics in a precocial bird: the ecology and evolution of brood parasitism in goldeneyes. PhD Dissertation, University of British Columbia.
Eadie JM, Kehoe FP, and Nudds TM. 1988. Pre-hatch and post-hatch brood amalgamation in North American Anatidae: A review of hypotheses. Canadian Journal of Zoology, 66: 1709–1721.
Eadie JM, and Savard JPL. 2015. Breeding systems, spacing behavior and reproductive behavior in sea ducks. In Ecology and conservation of North American sea ducks. Studies in Avian biology. Edited by JPL Savard, DV Derksen, D Esler, and J Eadie. CRC Press, New York, NY. pp. 367–417.
Eadie JM, Smith JNM, Zadworny D, Kühnlein U, and Cheng K. 2010. Probing parentage in parasitic birds: An evaluation of methods to detect conspecific brood parasitism using goldeneyes Bucephala islandica and B. clangula as a test case. Journal of Avian Biology, 41: 163–176.
Emlen ST, and Wrege PH. 1986. Forced copulations and intra-specific parasitism: Two costs of social living in the White-fronted Bee-eater. Ethology, 71: 2–29.
Fletcher DJ. 2012. Estimating overdispersion when fitting a generalized linear model to sparse data. Biometrika, 99: 230–237.
Gibbons DW. 1986. Brood parasitism and cooperative nesting in the moorhen, Gallinula chloropus. Behavioral Ecology and Sociobiology, 19: 221–232.
Gimenez O, Lebreton J-D, Choquet R, and Pradel R. 2018. R2ucare: An r package to perform goodness-of-fit tests for capture–recapture models. Methods in Ecology and Evolution, 9: 1749–1754.
Grueber CE, Nakagawa S, Laws RJ, and Jamieson IG. 2011. Multimodel inference in ecology and evolution: challenges and solutions. Journal of Evolutionary Biology, 24: 699–711.
Hepp GR, Kennamer RA, and Harvey WF. 1990. Incubation as a reproductive cost in female Wood Ducks. Auk, 107: 756–764.
Heusmann HW, Bellville RH, and Burrell RG. 1980. Further observations on dump nesting by wood ducks. Journal of Wildlife Management, 44: 908–915.
Jaatinen K, Lehtonen J, and Kokko H. 2011. Strategy selection under conspecific brood parasitism: An integrative modeling approach. Behavioral Ecology, 22: 144–155.
Jamieson IG, McRae SB, Simmons RE, and Trewby M. 2000. High rates of conspecific brood parasitism and egg rejection in Coots and Moorhens in ephemeral wetlands in Namibia. Auk, 117: 250–255.
Jetz W, Sekercioglu CH, and Böhning-Gaese K. 2008. The worldwide variation in avian clutch size across species and space. PLoS Biology, 6: e303–2657.
Laake JL. 2013. RMark: An R interface for analysis of capture-recapture data with MARK. AFSC Processed Rep 2013–01, 25 pp. Alaska Fish. Sci. Cent., NOAA, Natl. Mar. Fish. Serv., 7600 Sand Point Way NE, Seattle WA 98115.
Laake J. 2014. RMark: R code for MARK analysis. R package version 2.1.3. [online]: Available from cran. r-project.org/web/packages/RMark/index.html.
Lank DB, Rockwell RF, and Cooke F. 1990. Frequency-dependent fitness consequences of intraspecific nest parasitism in Snow Geese. Evolution, 44: 1436–1453.
Larsson K, Tegelstrom H, and Forslund P. 1995. Intraspecific nest parasitism and adoption of young in the barnacle goose - effects on survival and reproductive-performance. Animal Behaviour, 50: 1349–1360.
Lessels CM. 1986. Brood size in Canada Geese: a manipulation experiment. Journal of Animal Ecology, 55: 669–689.
Lombardo MP, Power HW, Stouffer PC, Romagnano LC, and Hoffenberg AS. 1989. Egg removal and intraspecific brood parasitism in the European starling (Sturnus vulgaris). Behavioral Ecology and Sociobiology, 24: 217–223.
Lyon BE. 1993. Tactics of parasitic American coots: host choice and the pattern of egg dispersion among host nests. Behavioral Ecology and Sociobiology, 33: 87–100.
Lyon BE, and Eadie JM. 2008. Conspecific brood parasitism in birds: a life-history perspective. Annual Review of Ecology, Evolution, and Systematics, 39: 343–363.
Lyon BE, and Eadie JM. 2017. Why do birds lay eggs in conspecifics’ nests? In Avian brood parasitism. Edited by M Soler.Springer, New York, NY. pp. 105–123.
Lyon BE, Hochachka WM, and Eadie JM. 2002. Paternity-parasitism trade-offs: a model and test of host-parasite cooperation in an avian conspecific brood parasite. Evolution, 56: 1253–1266.
Mallory ML. 2015. Site fidelity, breeding habitats, and the reproductive strategies of sea ducks. In Ecology and conservation of North American sea ducks. Studies in Avian biology. Edited by JPL Savard, DV Derksen, D Esler, and J Eadie. CRC Press, New York, NY. pp. 337–364.
McRae SB. 1995. Temporal variation in responses to intraspecific brood parasitism in the moorhen. Animal Behaviour, 49: 1073–1088.
McRae SB. 1996. Brood parasitism in the Moorhen: brief encounters between parasites and hosts and the significance of an evening laying hour. Journal of Avian Biology, 27: 311–320.
McRae SB. 1998. Relative reproductive success of female moorhens using conditional strategies of brood parasitism and parental care. Behavioral Ecology, 9: 93–100.
McRae SB. 2011. Conspecific brood parasitism in the tropics: an experimental investigation of host responses in common moorhens and American purple gallinules. Ecology and Evolution, 1: 317–329.
McRae SB, and Burke T. 1996. Intraspecific brood parasitism in the moorhen: parentage and parasite-host relationships determined by DNA fingerprinting. Behavioral Ecology and Sociobiology, 38: 115–129.
Milonoff M, and Paananen P. 1993. Egg formation, brood survival, and cost of reproduction as clutch size determining factors in common goldeneyes. Auk, 110: 943–946.
Nakagawa S, and Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: A practical guide for biologists. Biological Reviews, 85: 935–956.
Noel K, Titman RD, and Craik SR. 2021. No support for relatedness and kin selection to explain high rates of conspecific brood parasitism in colonial Red-breasted Mergansers. Canadian Journal of Zoology, 99: 435–441.
Nur N. 1984. The consequences of brood size for breeding Blue Tits. I. Adult survival, weight change and the cost of reproduction. Journal of Animal Ecology, 53: 479–496.
Odell NS, and Eadie JM. 2010. Do Wood Ducks use the quantity of eggs in a nest as a cue to nest’s value? Behavioral Ecology, 21: 794–801.
Paasivaara A, Rutila J, Pöysä H, and Runko P. 2010. Do parasitic common goldeneye Bucephala clangula choose nests on the basis of host traits or nest site traits? Journal of Avian Biology, 41: 662–671.
Pöysä H. 2003. Low host recognition tendency revealed by experimentally induced parasitic egg laying in the common goldeneye (Bucephala clangula). Canadian Journal of Zoology, 81: 1561–1565.
Pöysä H, Eadie JM, and Lyon BE. 2014. Conspecific brood parasitism in waterfowl and cues parasites use [special issue]. Wildfowl, 4:192–219.
Pradel R, Wintrebert CMA, and Gimenez O. 2003. A proposal for a goodness-of-fit test to the Arnason-Schwarz multisite capture-recapture model. Biometrics, 59: 43–53.
R Core Team. 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [online]: Available from R-project.org/.
Reichart LM, Anderholm S, Muñoz-Fuentes V, and Webster MS. 2010. Molecular identification of brood-parasitic females reveals an opportunistic reproductive tactic in ruddy ducks. Molecular Ecology, 19: 401–413.
Robertson GJ, Watson MD, and Cooke F. 1992. Frequency, timing, and costs of intraspecific nest parasitism in the common eider. Condor, 94: 871–879.
Rohwer FC, and Freeman S. 1989. The distribution of conspecific nest parasitism in birds. Canadian Journal of Zoology, 67: 239–253.
Rohwer FC, and Heusmann HW. 1991. Effects of brood size and age on survival of female Wood Ducks. Condor, 93: 817–824.
Roy Nielsen C, Parker PG, and Gates RJ. 2006a. Intraspecific nest parasitism of cavity-nesting wood ducks: costs and benefits to hosts and parasites. Animal Behaviour, 72: 917–926.
Roy Nielsen CL, Gates RJ, and Parker PG. 2006b. Intraspecific nest parasitism of Wood Ducks in natural cavities: Comparisons with nest boxes. Journal of Wildlife Management, 70: 835–843.
Sayler RD. 1992. Ecology and evolution of brood parasitism in waterfowl. In Ecology and management of breeding waterfowl. Edited by BDJ Batt, AD Afton, MG Anderson, CD Ankney, DH Johnson, JA Kadlec and GL Krapu. University of Minnesota Press, Minneapolis, MN. pp. 290–322.
Semel B, Sherman PW, and Byers SM. 1988. Effects of brood parasitism and nest-box placement on wood duck breeding ecology. Condor, 90: 920–930.
Sorenson MD. 1991. The functional significance of parasitic egg laying and typical nesting in redhead ducks: an analysis of individual behaviour. Animal Behaviour, 42: 771–796.
Sorenson MD. 1997. Effects of intra- and interspecific brood parasitism on a precocial host, the canvasback, Aythya valisineria. Behavioral Ecology, 8: 153–161.
Spurr EB, and Milne E. 1976. Factors affecting laying date in the Common Eider. Wildfowl, 27: 107–109.
Stoffel MA, Nakagawa S, and Schielzeth H. 2017. rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods of Ecology Evolution, 8: 1639–1644.
Tariel J, Longo G, Quiros A, Crane NL, Tenggardjaja K, Jackson A, Lyon B, and Bernardi G. 2019. Alloparental care in the sea: brood parasitism and adoption within and between two species of coral reef Altrichthys damselfish? Molecular Ecology, 28: 4680–4691.
Thimot NJ, Titman RD, Elliott KH, and Craik SR. 2020. Conspecific brood parasitism in an upland-nesting bird: cues parasites use to select a nest. Behavioral Ecology and Sociobiology, 74: 27.
Tucker AM, Dyer RJ, Huber SK, and Bulluck LP. 2016. Opportunistic conspecific brood parasitism in a box-nesting population of prothonotary warblers (Protonotaria citrea). Auk, 133: 298–307.
Waldeck P, Hagen JI, Hanssen SA, and Andersson M. 2011. Brood parasitism, female condition, and clutch reduction in the common eider Somateria mollisima. Journal of Avian Biology, 42: 231–238.
Waltho C, and Coulson J. 2015. The common eider. Poyser, London, UK.
Weller MW. 1957. An automatic nest-trap for waterfowl. Journal of Wildlife Management, 21: 456–458.
Westerskov K. 1950. Methods for determining the age of game bird eggs. Journal of Wildlife Management, 14: 56–67.
White GC, and Burnham KP. 1999. Program MARK: Survival estimation from populations of marked animals. Bird Study, 46: S120–S139.
Yom-Tov Y. 2001. An updated list and some comments on the occurrence of intraspecific nest parasitism in birds. Ibis, 143:133–143.
Young AD, and Titman RD. 1986. Costs and benefits to red-breasted mergansers nesting in tern and gull colonies. Canadian Journal of Zoology, 64: 2339–2343.
Young AD, and Titman RD. 1988. Intraspecific nest parasitism in red-breasted mergansers. Canadian Journal of Zoology, 66:2454–2458.
Zink AG. 2000. The evolution of intraspecific brood parasitism in birds and insects. American Naturalist, 155:395–405.
Supplementary material
Supplementary Material 1 (DOCX / 266 KB)
- Download
- 266.20 KB
Information & Authors
Information
Published In

FACETS
Volume 6 • Number 1 • January 2021
Pages: 2155 - 2176
Editor: John Eadie
History
Received: 19 July 2021
Accepted: 20 October 2021
Version of record online: 23 December 2021
Copyright
© 2021 Craik et al. This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Data Availability Statement
All relevant data are within the paper and in the Supplementary Material.
Key Words
Sections
Subjects
Authors
Author Contributions
SRC and RDT conceived and designed the study.
SRC and RDT performed the experiments/collected the data.
SRC, AMC, GJR, and SEG analyzed and interpreted the data.
SRC, RDT, and MLM contributed resources.
All drafted or revised the manuscript.
Competing Interests
Dr. Mark Mallory is a subject editor for FACETS.
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Shawn R. Craik, Rodger D. Titman, Anna M. Calvert, Gregory J. Robertson, Mark L. Mallory, and Sarah E. Gutowsky. 2021. Host traits and lifetime fitness costs of being parasitized in red-breasted mergansers. FACETS.
6: 2155-2176.
https://doi.org/10.1139/facets-2021-0104
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
Cited by
1.
