We found clear evidence of niche partitioning on multiple dimensions within shorebird species assemblages at small coastal sites in New Brunswick, Canada, during migratory staging. Environmental heterogeneity at coastal staging sites, coupled with differences among species in morphology and foraging behaviour, creates opportunities for partitioning of resources and allows a diverse shorebird assemblage to coexist.
Spatial segregation
Foraging microhabitat is an important dimension for segregation among coexisting species (
Burger et al. 1977;
Pöysä 1983;
Davis and Smith 2001;
Kent and Sherry 2020) as habitat influences the type and availability of prey (
VanDusen et al. 2012) as well as the efficacy of foraging behaviours (
Finn et al. 2008). The most prominent foraging microhabitats on the tidal flats at PCAP and CJ are tide pools, which created clear spatial boundaries between species that are and are not tall enough to forage in standing water (
Burger et al. 1977). Although tide pools in the Northumberland Strait often attracted multispecies flocks of shorebirds, this size division of available habitat was evident. Short-billed Dowitchers and Yellowlegs could access prey in deeper water due to their greater bill and tarsal lengths (
Baker 1979) and were more abundant in tide pools than the smaller species. Yellowlegs took advantage of their broad range of exploitable water depths by foraging both in and out of tide pools, whereas Short-billed Dowitchers almost exclusively foraged in tide pools. This is likely due to softer substrates in tide pools (
VanDusen et al. 2012), which make prey more accessible for tactile foragers such as the Short-billed Dowitcher (
Mouritsen and Jensen 1992;
Finn et al. 2008). Tide pools may be especially attractive for tactile foragers at coastal sites as sediments are often sandy and dense (
Davis 2019). Standing water also prolongs surface activity of invertebrates (
VanDusen et al. 2012), which may explain why all shorebirds in this study used tide pools to a certain extent. Semipalmated Sandpipers and Semipalmated Plovers were too small to forage in deep water (
Novcic 2016) and were clearly more abundant outside of tide pools. However, even they were attracted to the edges of tide pools where the water was shallow, perhaps because of increased invertebrate surface activity (
VanDunsen et al. 2012). Least and White-rumped Sandpipers were not abundant enough for analysis of their microhabitat preference; however, given their size, it is likely that they foraged more frequently outside of tide pools like Semipalmated Sandpipers and Semipalmated Plovers.
Spatial segregation between wet and dry microhabitats has been observed at other migratory stopover areas (e.g.,
Davis and Smith 2001) but is less prominent at sites with limited habitat diversity (
Novcic 2016). Therefore, heterogeneity of structural habitat, such as the presence and absence of tide pools, facilitates spatial partitioning of different shorebird species into microhabitat niches. We found evidence of significant spatial heterogeneity of prey availability within coastal sites, and there is a correlation between species composition of foraging flocks and the available prey community where they foraged. As such, if shorebird species target different prey items, prey heterogeneity may contribute to spatial segregation at coastal sites. However, given that some species that overlap in foraging microhabitat had significantly different diet niches (e.g., Semipalmated Sandpipers and Semipalmated Plovers), there is little evidence to suggest that shorebirds target microhabitats within a site based on prey availability or that spatial segregation is driving variation in diet niches. Morphology and foraging behaviour are more likely facilitators of diet variation, as has been suggested for similar species at different stopover sites (
Davis and Smith 2001).
Behaviour and diet segregation
Foraging behaviour is a widely studied niche dimension in birds (e.g.,
Baker and Baker 1973;
Davis and Smith 2001;
Novcic 2014,
2016;
Choi et al. 2017). However, unless prey are distributed in a way that restricts their availability to particular foraging behaviours, it may not reflect prey taxon-level resource partitioning, as large behavioural differences often translate to only small differences in diet (
Kent and Sherry 2020). The main difference between pecking and probing foraging behaviours is the depth of consumed prey (
Dit Durell 2000), which largely depends on bill morphology, as birds with longer bills can access a wider range of probing depths. Therefore, shorebird species foraging at different sediment depths target spatially segregated prey items, though the prey taxa in their diets may overlap unless these taxa are segregated by depth. For example, gastropod species typically dwell on or near the sediment surface (
Huxham et al. 1995;
Chandrashkara and Frid 1998), while other invertebrate taxa, including bivalves and polychaete worms, can be found at a range of sediment depths (
Henriksen et al. 1983;
Zwarts and Wanink 1989;
Touhami et al. 2018). Within the taxonomic levels to which we classified burrowing invertebrates for diet niche models in this study, individuals can be found at a wide range of depths, sometimes spanning 0 to over 10 cm (
Zwarts and Wanink 1989;
Davey 1994). Larger prey also tend to burrow deeper in muddy or sandy intertidal sites (
Zwarts and Wanink 1989;
Coulthard and Hamilton 2011;
Touhami et al. 2018). Thus, long-billed shorebird species, which in our study were larger than the short-billed plovers and small Calidrids (and therefore could consume larger prey items), may have had access to prey items that their smaller competitors could not have consumed. Variation in foraging behaviour therefore reveals a potential for niche partitioning via foraging depth that could not be detected by diet examination alone. As such, it is important to pair behavioural observations with diet analyses when attempting to explain resource partitioning.
We found significant differences in the foraging behaviours and diets of shorebird species during staging in the Northumberland Strait. Short-billed Dowitchers had very high probing rates and low pecking rates, resulting in little behavioural overlap with other species in this study. Long bills, such as that of the Short-billed Dowitcher, are adapted for probing deep into the sediment (
Barbosa and Moreno 1999), and thus, pecking behaviours are rarely observed (
Baker and Baker 1973;
Novcic 2016). This specialized foraging behaviour has been observed in Dowitchers across their migratory range (
Baker 1979;
Davis and Smith 2001;
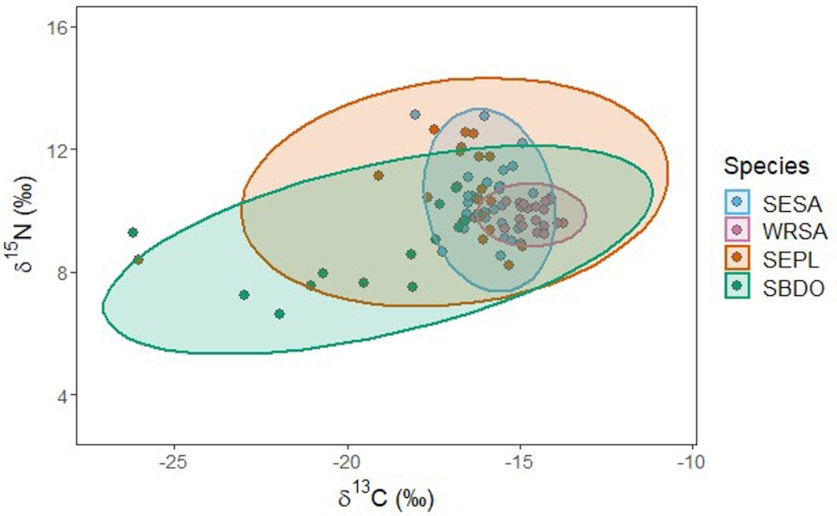
Novcic 2016), and likely is necessary to reach prey buried in the sediment under standing water. At our site, this behaviour would have provided these birds with access to prey that most other species could not reach. Analyses of plasma isotopes suggested that Short-billed Dowitchers consumed a broad diet, and their lower range of δ
15N values suggests they consumed prey from lower trophic levels than the other species. The diet niche of Short-billed Dowitchers had some overlap with other species; however, their behavioural specialization and limited foraging microhabitat likely result in a realized niche that has little competitive overlap with the other shorebird species on the Northumberland Strait.
Semipalmated Plovers also had unique foraging behaviour. They pecked more than probed, and overall, their foraging rates were low compared with other species. Semipalmated Plovers are visual foragers that spend more time moving and looking for prey items than making foraging attempts (
Nol 1986;
Ouellette 2021), which results in lower foraging rates than species that peck or probe steadily while walking (
Baker and Baker 1973).
MacKellar (2018) found that Semipalmated Plovers at similar coastal sites in northeastern New Brunswick had lower foraging rates than Semipalmated Sandpipers feeding in the same area. Given their short bills and pecking behaviour, the dietary niche of Semipalmated Plovers is likely limited to invertebrates dwelling on or near the sediment surface (
Dit Durell 2000).
Rose et al. (2016) found that Semipalmated Plovers foraged opportunistically and consumed a broad range of invertebrate prey items across various foraging sites on the nonbreeding grounds. This dietary opportunism was reflected in a broad isotopic niche and likely balances their limited range of accessible prey depth. Semipalmated Plovers likely experienced substantive competitive overlap only with the Calidrid sandpipers in the Northumberland Strait due to their similarity in foraging microhabitat. Further, Semipalmated Plovers at Petit-Cap concentrate feeding during daylight hours, while sandpipers continue to forage nocturnally (
Ouellette 2021), suggesting an additional level of temporal segregation that would reduce resource competition among these species. As such, Semipalmated Plovers’ broad diets and narrow range of foraging behaviours combined with diurnal foraging trends may allow them to coexist with sandpipers to the extent observed in the Northumberland Strait.
Unlike Short-billed Dowitchers and Semipalmated Plovers, Yellowlegs pecked and probed at similar rates while foraging. This resulted in foraging behaviour that differed from all but the White-rumped Sandpiper. Yellowlegs are tall shorebirds and often forage in standing water (
Danufsky and Colwell 2003). Yellowlegs in the Northumberland Strait foraged both in and out of tide pools. Sediment coarseness affected pecking and probing rates of foraging shorebirds, with coarser sediment inhibiting foraging efficiency, as has been seen in other studies (
Danufsky and Colwell 2003). These coarser sediments tended to be drier, while wetter and more penetrable sediments supported more probing behaviours. Therefore, Yellowlegs may adjust their foraging behaviour for the sediment conditions they encounter in different microhabitats, resulting in similar mean pecking and probing rates. Yellowlegs’ broad habitat use likely resulted in competitive overlap with both Short-billed Dowitchers and the smaller sandpipers and plovers. We did not estimate diet of Yellowlegs; however,
Andrei et al. (2009) found that Yellowlegs consumed broad diets at stopover sites in the southern United States. Niche complementarity predicts that Yellowlegs should exhibit niche specialization on some dimension to compensate for competitive overlap (
Schoener 1974). However, it is possible that Yellowlegs forego foraging specialization and instead use a generalist staging strategy to exploit the full range of available resources at coastal sites.
Semipalmated Sandpipers, Least Sandpipers, and White-rumped Sandpipers did not have significantly different foraging behaviour. These species are congeners and are morphologically similar (
Thomas et al. 2004), which likely plays a part in their similar behaviour (
Barbosa and Moreno 1999;
Dit Durell 2000;
Norazlimi and Ramli 2015). All three sandpiper species used both pecking and probing behaviours but had higher pecking rates than probing rates. Resource competition is likely high among the sandpiper species; however, differences in diet breadth may have compensated for spatial and behavioural overlap. The diet of White-rumped Sandpipers was considerably narrower than that of Semipalmated Sandpipers. On their wintering grounds, White-rumped Sandpipers consume high proportions of the most abundant prey items (
de los Angeles Hernandez and Bala 2007), which suggests they modify their diet to maximize foraging efficiency at different sites across their migratory range. The diet niche of Semipalmated Sandpipers in our study was also narrow compared with niches of Short-billed Dowitchers and Semipalmated Plovers; however, Semipalmated Sandpipers are considered generalist foragers (
Gerwing et al. 2016) and have been found to opportunistically target the most available prey when more favourable prey items are unabundant (
MacDonald et al. 2012). The heterogeneity of habitat and prey assemblages in the Northumberland Strait likely allows Semipalmated and White-rumped Sandpipers to opportunistically target the specific prey taxa that they can consume most efficiently, thus limiting dietary overlap with Dowitchers and Plovers.