Introduction
The American common eider subspecies,
Somateria mollissima dresseri, breeds on coastal islands of northeastern North America from approximately 41°N–54°N and 53°W–70°W (
Lock 1986;
Noel et al. 2021). Once harvested to near extirpation in Nova Scotia, common eider was successful in rebuilding populations such that the species became a prized game bird after the “Migratory Bird Convention Act” and its prohibitions to allow regulated hunting were passed in 1916 (
Allen 2000;
Rothe et al. 2015). Although numbers of American common eider have declined recently (
Canadian Wildlife Service Waterfowl Committee 2020), the pattern is not uniform across the subspecies’ range, with increasing numbers of breeding birds in the north, and stable or decreasing numbers in the south (
Bowman et al. 2015;
Chardine 2015;
Noel et al. 2021).
Milton et al. (2016) hypothesized that the low survival for Nova Scotia breeding female American common eider (
S = 0.827 ± 0.023) and corresponding local breeding population declines were consistent with population dynamics of long-lived sea ducks (
Flint 2015), and have been observed with other common eider subspecies exhibiting low adult survival (e.g.,
Öst et al. 2016). Moreover,
Milton et al. (2016) considered that higher male eider survival rates (
S = 0.915 ± 0.021) and similar recovery rates with females (
f = 0.013) in Nova Scotia suggested that lower female survival was attributable to factors other than hunting, such as predation on females at breeding colonies (e.g., mustelids, bald eagle (
Haliaeetus leucocephalus);
Nordström et al. 2002;
Waltho and Coulson 2015). Analyses of recruitment and population growth rates (
Giroux et al. 2021) using capture and recapture records of breeding females across the
Somateria mollissima dresseri range report both declining populations and low recruitment rates in Nova Scotia colonies. Among several potential drivers, low recruitment rates could reflect the effects of natural, dynamic processes of vegetation change (
Clarkson et al. 2014), altering the quality of nesting habitat. Habitat change may also include effects of double-crested cormorant (
Phalacrocorax auritus) faeces, when this species establishes a colony on a nesting island. The faeces dramatically alter overhead and ground vegetation (
Milton et al. 1995;
Kolb et al. 2012;
Hebert et al. 2014), reducing the quality and/or amount of available breeding habitat, and thereby exposing nests, ducklings, and incubating female eiders to predators and adverse weather conditions.
Common eiders nest on the ground, breeding in colonies of a few to hundreds of pairs on offshore islands, presumably to avoid mammalian predators (
Goudie et al. 2000;
Chaulk et al. 2007). Numerous studies have documented eider preference for some type of cover (e.g.,
Gross 1944;
Schamel 1977;
Laurila 1989;
Woolaver 1997). Even in open landscapes, breeding eiders cluster around features such as tufts of grass or rocks (
Gerell 1985;
Fast et al. 2007). While most studies cite predator avoidance as a main driver of nest site selection, eiders may also choose sites with cover that affords thermoregulatory benefits to save on energetic costs (e.g.,
Schamel 1977;
van Dijk 1986;
Shutler et al. 1998;
Fast et al. 2007). As the sole incubators of eggs, female common eiders take only extremely short and infrequent recesses from their nests (
Bolduc and Guillemette 2003a), and may lose up to 40% of their body mass during egg laying and incubation (
Korschgen 1977;
Bolduc and Guillemette 2003b;
Are Hanssen et al. 2003). Further,
Kilpi and Lindström (1997) found that eider hens incubating in exposed habitats laid fewer eggs and lost weight at a faster rate than those incubating in sheltered areas. In a separate study, eiders protected under nesting structures were heavier at mid-incubation than those incubating at open sites (
Fast et al. 2007).
Due to their high nest site fidelity and natal philopatry (
Goudie et al. 2000;
Öst et al. 2011;
Ekroos et al. 2012), common eiders are vulnerable to ecological traps when conditions at breeding locations change (
Robertson and Hutto 2006;
Igual et al. 2007;
Ekroos et al. 2012). Eiders may continue to return to previous nesting sites where they were once successful, even though the conditions that led to previous success have changed. Colonial bird surveys by Nova Scotia government biologists within the Eastern Shore Islands Wildlife Management Area (ESIWMA) have observed dramatic changes in the vegetation structure over three decades, as some islands progressed from being fully treed, to dead and fallen timber, to shrub- and grass-covered with little regeneration of shrubs or trees (G.R. Milton, unpublished data). This study investigates habitat change as a possible driver of change in American common eider breeding colony size and female survival 20 years after
Woolaver (1997) classified and quantified the types of habitat available on islands used by nesting female eiders in the ESIWMA. We predicted that eiders breeding in the ESIWMA were increasingly nesting under suboptimal breeding conditions, leaving them exposed to predators and increased physiological stress. We compare habitat availability versus use by American common eiders in 1992–1993 and 2013 and the effect of habitat structure to moderate temperature for incubating hens through shading from the sun as the breeding season progressed.
Study area
The islands found along the Atlantic coast of Nova Scotia, Canada, represent a significant breeding ground for many colonial nesting marine birds, including black guillemot (Cepphus grylle), Arctic tern (Sterna paradisaea), herring gull (Larus smithsoniansus), great black-backed gull (Larus marinus), Leach’s storm-petrel (Hydrobates leucorhous), great cormorant (Phalacrocorax carbo), double-crested cormorant (Phalacrocorax auritus), and American common eider. For cormorants, they initially nested in trees on the islands, but eventually their guano killed the vegetation, and if the colony did not move, it transitioned to ground-nesting. Cormorants have nested on several of the islands over the years (e.g., Camp, Speck, Sandy, Pancake).
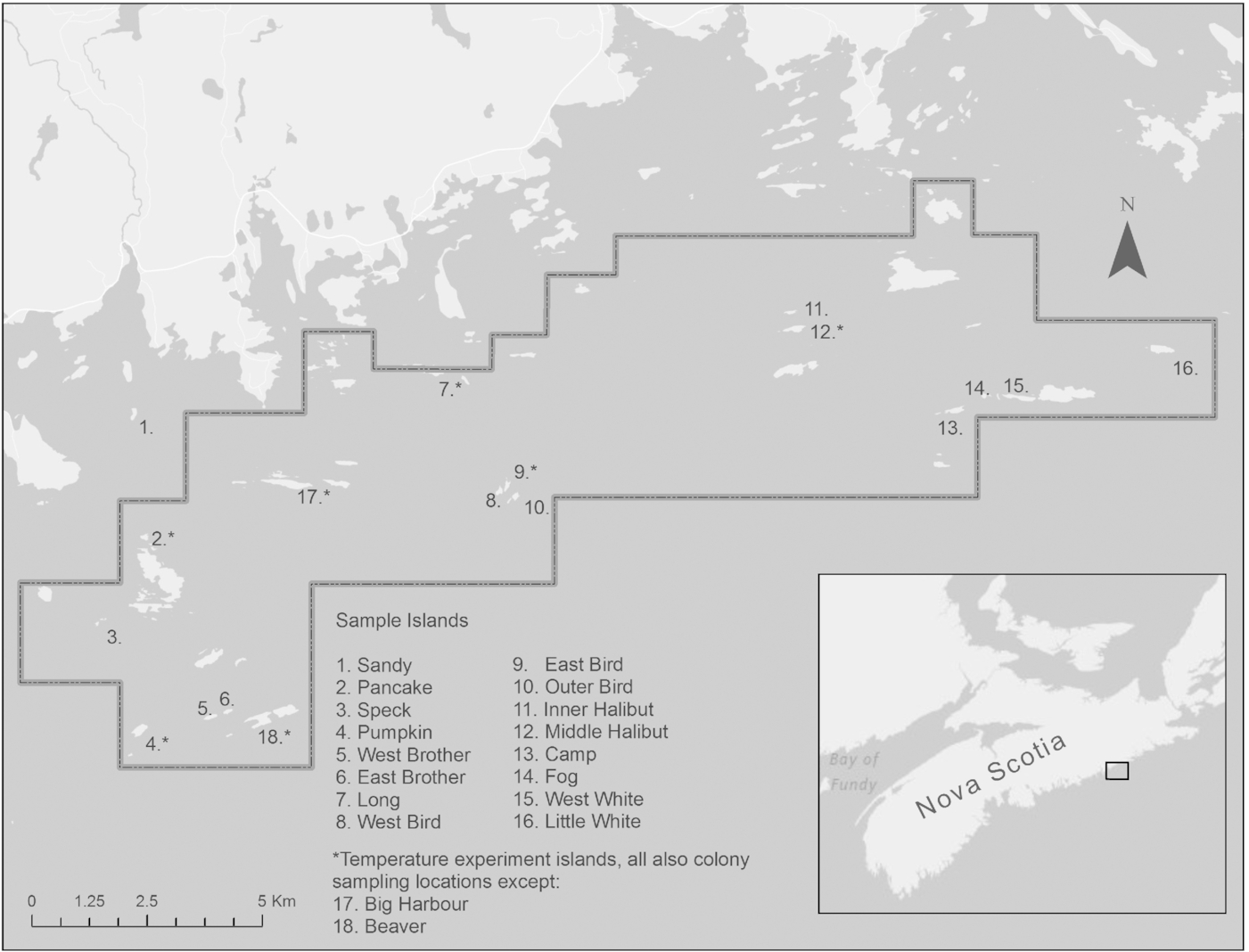
In 1976, a subset of islands between Sheet Harbour Passage (44.86°N, −62.47°W) and Marie Joseph (44.97°N, −62.08°W), encompassing approximately 60 vegetated and nonvegetated islands, islets, and ledges, and thought to host 25% of the provincial breeding population of American common eider in Nova Scotia, were designated as the ESIWMA (
Payne 1977). Although hunting and trapping are permitted in season, human disturbance on the islands is prohibited throughout the breeding season (April–August). This study focuses on a subset of 16 islands within the ESIWMA and immediately adjacent (
Fig. 1). Because of their long history of protection and study, these islands provide the most long-term and comprehensive data sets on eider colonies and island habitats in Nova Scotia.
Discussion
American common eider numbers in Nova Scotia have fluctuated greatly over the past century, from lows during unregulated harvest before 1916 to strong breeding numbers in the 1990s (
Bowman et al. 2015;
Noel et al. 2021), making it difficult to establish a value for an expected, sustainable baseline (
Soga and Gaston 2018) in the province. Nonetheless, the number of nesting eiders in the ESIWMA declined by >40% over the two decades since the 1992–1993 study by
Woolaver (1997), and expert opinion suggests this trend may have continued over the last decade (
Noel et al. 2021). Colony size on islands varied between the periods but was mostly negative, with dramatic losses of the large colonies; six islands supported 20 or fewer nests at the last census. For example, Middle Halibut Island, which formerly supported more than 300 nests, had only 23 nests in 2013; mammalian predators were using the island regularly since 2010 (G. Parsons, personal observation). The declining island breeding numbers were consistent with expected responses to low female survival (
Milton et al. 2016) and recruitment and population growth rates (
Giroux et al. 2021) within the Nova Scotia population. Although breeding effort within a population can vary from year to year (
Gilliland et al. 2005), and a perceived risk of reduced survival can deter breeding (
Coulson 1984;
Jaatinen et al. 2022), long-term data from the study site show gradual but steady declines in breeding numbers and colony sizes from the mid-1990s onwards (G.R. Milton, unpublished data). So, while a decline in breeding numbers does not necessarily mean an equivalent population decline, such a prolonged period of low reproductive effort would contribute to overall population decline. Continued monitoring could attempt to assess the propensity for nonbreeding in this population.
What was the main driver of these losses? One hypothesis was that hunting overharvest could result in the observed declines. However, this is not supported in the harvest data. Although female survival was ∼10% lower than that of males, banded males and females had similar recovery rates (
Milton et al. 2016). Additionally, males are actually favored in the American sport hunt for their striking plumage (
Rothe et al. 2015).
Another hypothesis for the declining breeding population and elevated mortality rate of females was that they may have been exposed to disease and contaminants. While
Ballard et al. (2017) found that Wellfleet Bay virus antibodies were present in 3.4% of birds sampled in Nova Scotia, these rates were equivalent to prevalence in Québec, where populations were not thought to be declining (
Bowman et al. 2015;
Chardine 2015;
Noel et al. 2021). There were also no observed large-scale die-offs that one might expect during a disease outbreak at a breeding colony (
Descamps et al. 2009;
Ballard et al. 2017). Moreover, contaminants were not thought to be contributing to the overall population decline observed in the ESIWMA, at least for toxic, nonessential trace elements, because observed concentrations in eggs were generally lower than that for most other eider populations in Canada (
Pratte et al. 2015).
Instead, an alternative hypothesis for lower breeding numbers was that altered physical characteristics of breeding sites, such as availability of cover, may be contributing to the breeding population decline in the ESIWMA. Both vegetation change, predation, and their interaction have been identified as contributing to dramatic declines in common eider populations in Europe (e.g.,
Ekroos et al. 2012). Like the European situation, in eastern North America north to coastal Greenland, increases in eagle abundance, and concomitant predation on eiders, is thought to influence eider numbers and behavior in recent years (
Merkel and Mosbech 2008;
Milton et al. 2016;
Allen et al. 2019), including at the ESIWMA (R. Milton, personal observation). While some of the variability in total vegetated area detected on all islands measured in 1992 and 2013 was likely attributable to measurement error (i.e., islands with <10% change), we observed some clear and dramatic changes. Overall reduction in vegetated cover across the archipelago was slight at 7.7%, but some islands experienced reductions of 17%, 32%, or even 79%. Three of the six islands that experienced >10% reduction in total vegetation had active cormorant colonies in 2013 (Camp, Sandy, and Speck Islands), and another was a former colony (Pancake Island). Cormorant guano deposited at these sites killed off vegetation, leaving bare, exposed ground, and standing or fallen deadwood at various stages of decomposition, as has been found in studies elsewhere (
Cuthbert et al. 2002;
Hebert et al. 2014). However, not all islands with cormorant colonies experienced declines. Cormorants nesting on the exposed bedrock on the periphery of the island (e.g., Little White and Outer Bird Islands) did not significantly reduce vegetative cover. Thus, the presence of cormorants alone is not necessarily a causal factor in habitat change; interactions with the type of habitat at the time of cormorant colonization (notably tree presence and use by nesting and roosting birds) appears key for determining influence on eider nesting.
Certain physical structures and geological make-up of each island may also be a factor in vegetation change, as three of the islands with dramatic changes were relatively small and low lying, increasing the proportion of vegetation edge exposed to the erosive forces of wind and waves (Pancake, Sandy, and Speck Islands). Islands with narrow strips or small, isolated patches of vegetation near the periphery were more susceptible to vegetation loss (Sandy, Speck, and West White Islands). For example, while Sandy Island supported nesting pairs of cormorants in 2013, it was also the only site that comprised principally deposited till and/or accumulated sediment, rather than bedrock, and thus appears to have been more susceptible to erosive forces with vegetation loss.
Erwin et al. (2011) also noted that habitat change, notably erosion and vegetation loss, contributed to substantial shifts in waterbird numbers in Chesapeake Bay, USA. With increased storm frequency and sea level rise projections predicted by climate change modelling for the region, islands along Nova Scotia’s Atlantic coast are likely to experience more erosion and loss of vegetation (
Molnar et al. 2021).
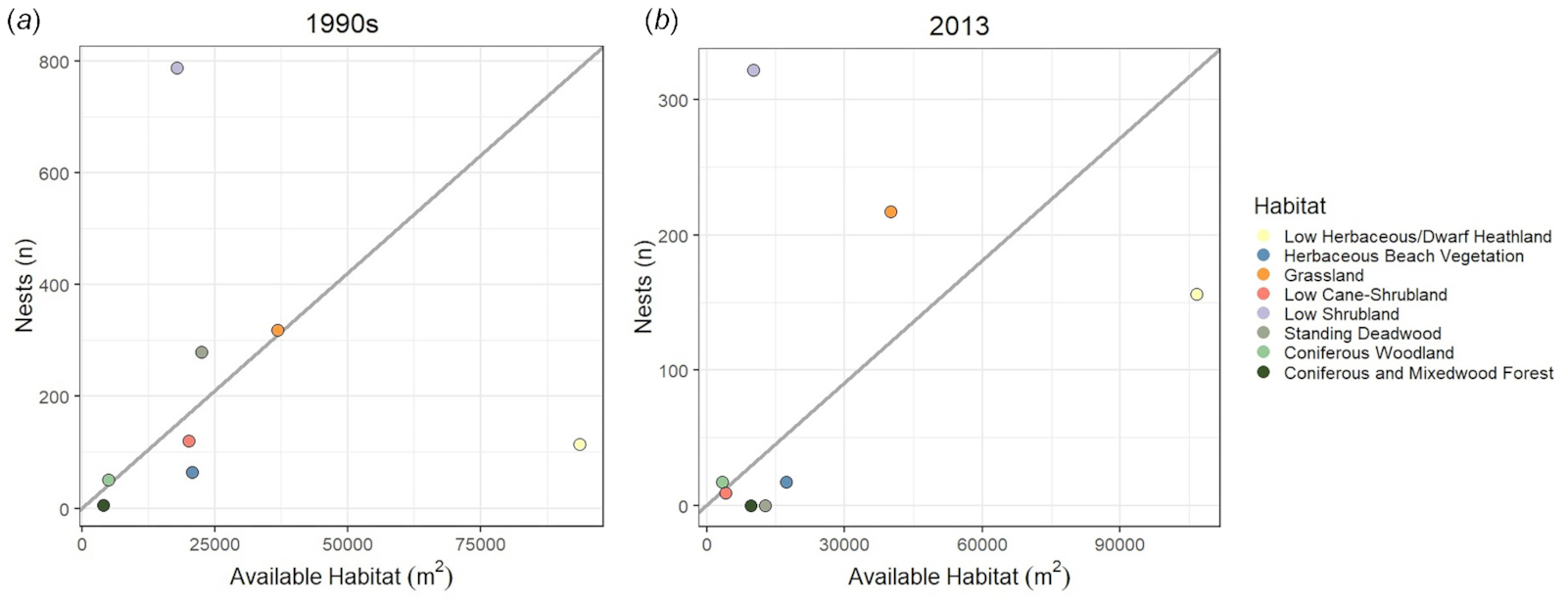
Changes in the coverage provided by the eight habitat types used by breeding eider in the ESIWMA clearly varied over approximately two decades. At least some of the changes may have been attributable to the establishment, occupation, and then abandonment of cormorant colonies, and subsequent habitat succession.
Milton et al. (1995) and
Woolaver (1997) posited that cormorant colonies may actually be beneficial to nesting American common eiders by destroying forest cover, which eiders seem to avoid, creating standing and fallen deadwood, and encouraging regeneration of Low Cane-Shrubland. This may have been the situation when cormorants were commonly nesting in softwood forests in the 1990s. However, by 2013, all cormorant colonies were on the ground, creating open barren areas or exposed rock covered in guano. Nonetheless, vegetation changes across islands were not consistent, with some islands experiencing local increases or decreases at odds with the overall trend for the archipelago. Areas initially covered in Low Cane-Shrubland and Standing (and fallen) Deadwood favored by nesting eiders in the 1990s (
Woolaver 1997) were largely converted to Low Herbaceous/Dwarf Heathland with some sparse raspberry cane growth and fallen deadwood by 2013. Some regeneration of forest cover had occurred at some sites where it was not completely lost to nesting cormorants. While Low Cane-Shrubland decreased consistently across islands (10/13 islands), at least two islands with recent cormorant colonies experienced increases in this cover type. Collectively, these findings suggest that after cormorants abandon colonies in forested areas, initial vegetation succession may provide favorable breeding habitat for nesting American common eiders, but this may be island-specific. However, the long-term data presented in this study indicate that later stages of succession, or succession in nonforested cover types, do not favor breeding eiders, but increase the area of cover types that breeding eiders tend to avoid.
Despite the overall decline in vegetation and the changes in overall availability of most cover types, breeding eider habitat selection was remarkably consistent between the 1990s and 2013. Observed changes in habitat selection may be attributed to overall changes in the relative availability of those same habitat types. Hens continued to heavily favor Low Shrubland, and strongly avoid Low Herbaceous/Dwarf Heathland. They rarely nested in Coniferous and Mixedwood Forest and Herbaceous Beach Vegetation, and used the relatively rare Coniferous Woodland in proportion to its availability. Low Cane-Shrubland was a relatively common habitat type in the 1990s but quite rare in 2013. Eiders continued to use this now scarce habitat type, resulting in an increase in nest density and the relative preference for this habitat type in 2013. With a decrease in availability of preferred Standing Deadwood and Low Shrubland from the 1990s and an increase in Low Herbaceous/Dwarf Heathland, eiders increased their relative use of Grassland in 2013.
The most favored habitat type, Low Shrubland, presumably offers concealment and thus reduced risk of predation, notably from avian predators (e.g.,
Öst et al. 2008), but also appears to offer some protection from less favorable weather conditions in terms of temperature moderation, with very few days reaching the maximum recorded temperature of 26.4 °C during the breeding season. This cover type also remained close to the overall mean temperature for all habitat types throughout the breeding season but warmed at a slower rate than the overall mean, offering some temperature moderation as the ambient temperature increased later in the season. This temperature moderation may reduce incubation costs associated with thermoregulation at the southern extent of this species’ range, and reduce risks of eggs overheating if the female is flushed from the nest (
Choate 1967).
Older and more experienced eider females are thought to breed earlier (
Korschgen 1977;
Schamel 1977) and to choose higher quality habitats (
Are Hanssen et al. 2003). Predation rates (
Woolaver 1997;
Ekroos et al. 2012) and incubation costs (
Kilpi and Lindström 1997;
Fast et al. 2007) differ based on cover type, with both predation pressure and incubation cost increasing in open habitats. Higher quality, experienced females are, therefore, expected to choose sites offering the best cover for maximizing fitness in the form of short- and long-term survival and reproductive success. Numerous studies have corroborated our findings that eiders prefer low shrub habitats despite their ability to nest in a variety of cover types (e.g.,
Gross 1944;
Gerell 1985;
van Dijk 1986), suggesting that these habitats offer maximum benefits to breeding females in terms of both survival and reproduction.
Consequently, we suggest that long-term conservation and management of sustainable eider colonies needs to consider dynamic habitat succession and availability, and the way these interact with threats like predation and disturbance, at least in forested island habitats like those in coastal Nova Scotia. Because island features may change through time, habitat managers should target protection and management of whole archipelagos, enacting regulations to minimize access and disturbance of nesting eiders (
Bolduc and Guillemette 2003a), and may want to consider deploying nest shelters (
Lusignan et al. 2010) to augment or sustain local colonies. Control of avian and mammalian predators may need to be considered (
Mawhinney et al. 1999;
Jaatinen et al. 2022), particularly if those predator populations are being subsidized by human industrial food sources (which may require direction management action as well;
Gutowsky et al. 2021). In some cases, management of eider harvest may also be required (
Rothe et al. 2015), although we note that human harvest is likely not a major factor in declining colony numbers in Nova Scotia (
Milton et al. 2016). Collectively, wildlife and habitat managers have a suite of options to help support local eider populations; the complexity of regional and local drivers of recent eider colony declines (
Noel et al. 2021) suggest that management solutions will likely require multiple actions.