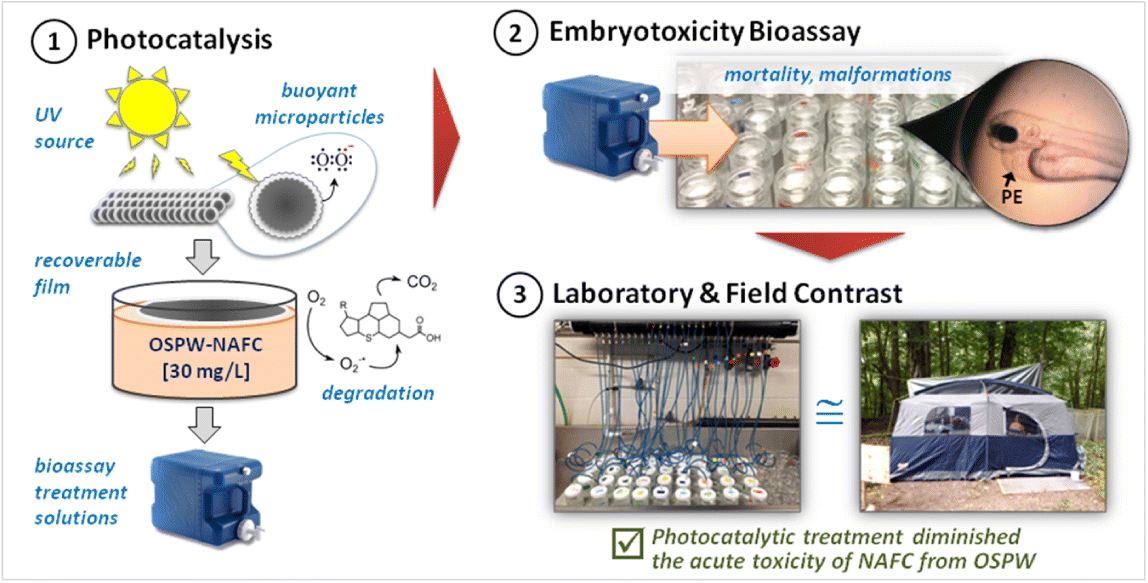
TiO2-mediated photocatalysis reduced the acute lethality of NAFC extracts
Passive photocatalytic treatment using TiO
2 microparticles reduced the acute toxicity of NAFC extracted from OSPW to developing FHM embryos (
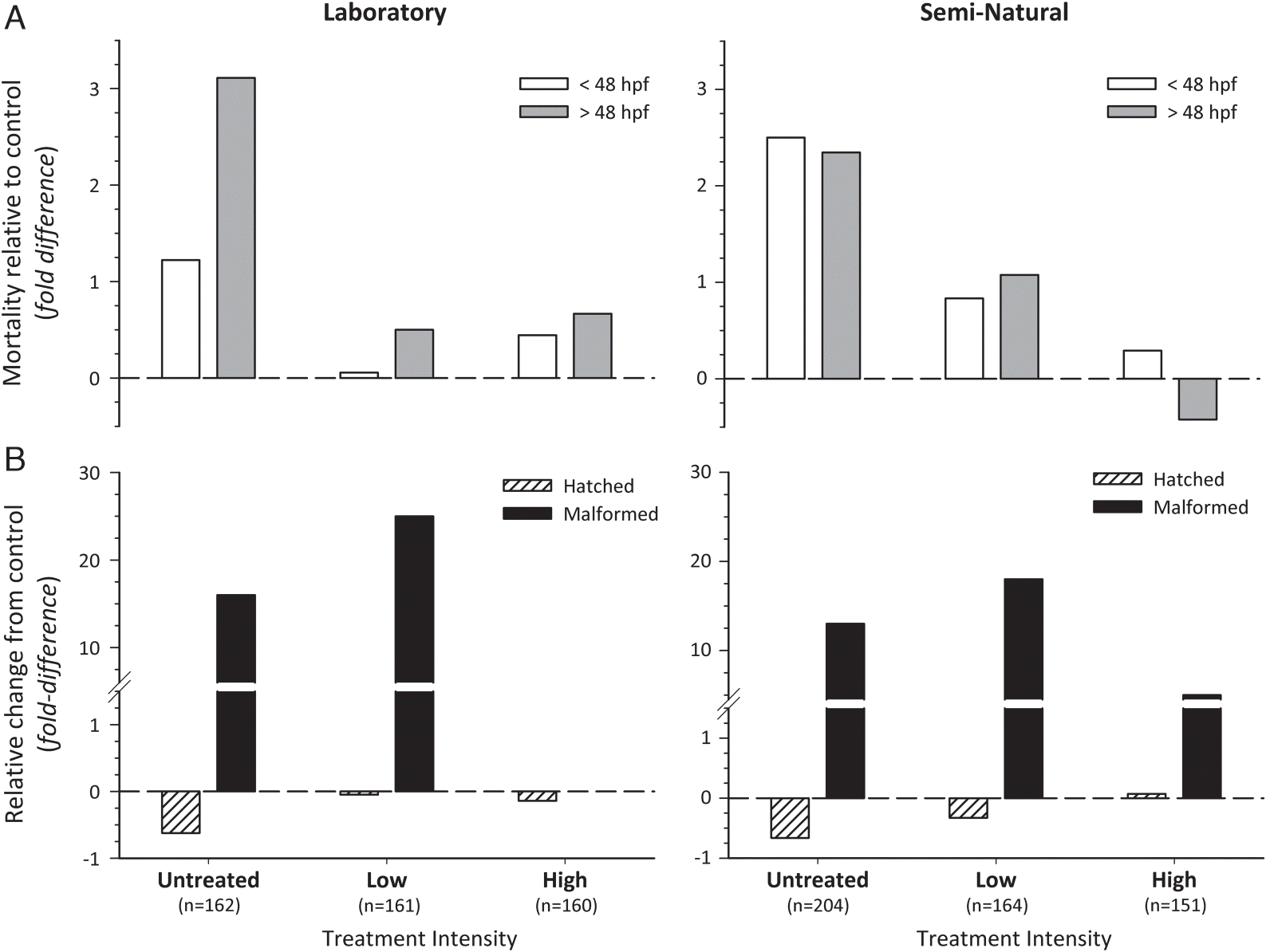
Fig. 1A). In untreated NAFC solutions, we observed a three-fold (i.e., ∼74% and 83% for laboratory and semi-natural experiment, respectively) increase in acute mortality relative to negative controls. In contrast, high-intensity photocatalysis of NAFC solutions resulted in almost complete elimination of NAFC-induced acute mortality. Low-intensity treatments approximately halved the acute mortality in developing FHM observed relative to untreated NAFC solutions, but notably, mortality in treated fish was overall higher than that observed in earlier experiments that assessed 48-h LC50 (8–21 mg/L) and EC50 (∼22–24 mg/L) for FHM with similar concentrations of NAFC (
Marentette et al. 2015a,
2015b). However, differences in NAFC quantification methods between these studies may account for the observed differences in FHM mortality. Mortality within respective treatments was comparable between laboratory and semi-natural experiments, bolstering confidence in the reproducibility of these findings outside of controlled settings.
The immediate toxicity observed with NAFC exposure can likely be attributed to nonspecific membrane disruption (i.e., acute narcosis), which arises from the surfactant properties of NAs (
Marentette et al. 2015b;
Morandi et al. 2015;
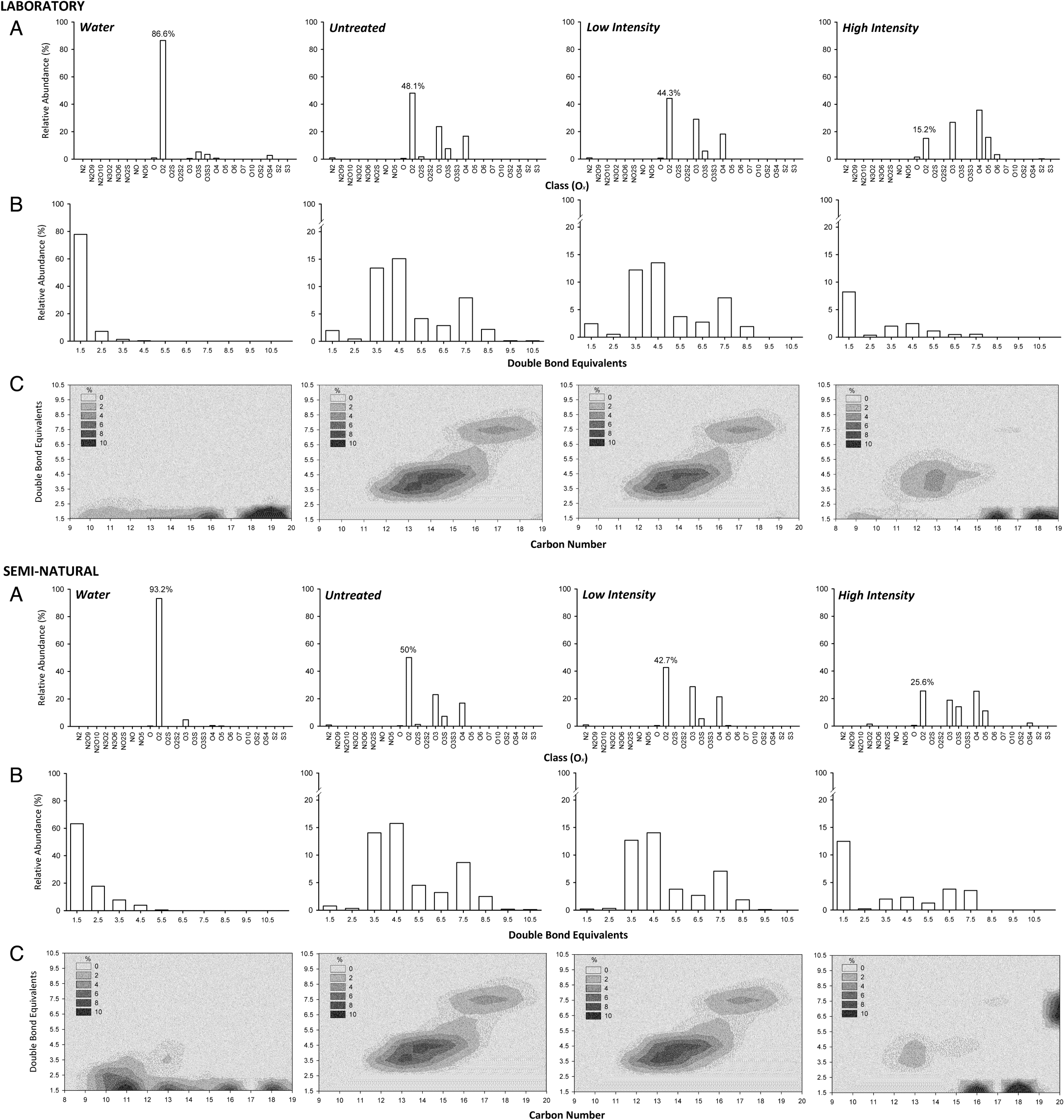
Yue et al. 2015). Here, the toxicity of NAFC appeared to be related to the relative proportions of lower numbered oxygen class (O
x), including classical (O
2) NAs and O
3 to O
4 compounds (
Fig. 2A). These have long been characterized as dominant components of fresh OSPW (
Han et al. 2008;
Headley et al. 2011,
2013;
Leshuk et al. 2018b), and toxicity in general decreases with increased oxygen content (
Frank et al. 2008,
2009). This coincides with our finding that acute mortality of FHM embryos decreased with increasing photocatalytic treatment intensity and increased abundance of O
4, O
5, and O
6 compounds, possibly related to the degradation of higher DBE components (
de Oliveira Livera et al. 2018).
Leshuk et al. (2018b) also reported a clear relationship between increased photocatalytic treatment intensity and increased oxygen content with high-intensity treatment of raw OSPW increasing average oxygen numbers from 2.5 to 4.3. We also observed that photocatalytic treatment of NAFC extracts from OSPW greatly reduced the proportions of similar sized O
x classes, growing the proportion of oxygen numbers above 3.5 (
Fig. 2B) and reducing the proportion of O
2 compounds with 13 to 18 carbons (
Fig. 2C). More specific detail of the chemical changes to particular NA classes resultant of this TiO
2-mediated photocatalytic treatment can be found in related work by
de Oliveira Livera et al. (2018).
Chronic toxicity of NAFC can be mitigated by high-intensity photocatalytic treatment
The relationship between the intensity of photocatalytic treatment of NAFC solutions and chronic toxicity experienced by developing fish is more nuanced than its outright lethality. Though the photocatalytic treatment intensity was proportional to hatch success, almost all treatments, irrespective of intensity, had more cardiovascular abnormalities than negative controls at the time of hatch (
Fig. 1B). The cardiovascular abnormalities we observed in FHM exposed to NAFC were predominantly (∼95%) pericardial edema with tube heart. Rearing environment appeared to influence the toxic responses of fish in high-intensity treatments, although the final concentration of NAFC following treatment was not identical between laboratory and field experiments, i.e., 2.5 and 3.9 mg/L, respectively. This effect is, perhaps not unexpected, as NAFC measured in our solutions following high-intensity treatment were comparable to previous estimates of LOEC (∼4 mg/L) for similar NAFC extracts (
Marentette et al. 2015a,
McQueen et al. 2016). These findings highlight the need for assessments of treatment methods for oil sands waste waters to consider both acute and chronic toxicity endpoints, as well as the test environment when considering safe release threshold concentrations for OSPW into surrounding receiving waters.
The proportion of FHM hatched with cardiovascular abnormalities that were exposed to NAFC solutions in our study are consistent with previous studies examining the toxicity of OSPW (
He et al. 2012) and NAFC (
Marentette et al. 2015a,
2015b). General cardiovascular abnormalities may be attributed to DNA damage caused by oxidative stress. Oxidative stress and the activation of the aryl hydrocarbon receptor (AhR) contribute to cellular damage in fish embryos, including cardiovascular abnormalities resulting from DNA damage (
Toomey et al. 2001;
Barron et al. 2004;
Marentette et al. 2017). OSPW contains other petroleum-related compounds, such as PACs, and that could contribute to toxicity of oil sands extracts (
Headley et al. 2013). Recent analyses of diluted bitumen toxicity to fish links PACs to oxidative stress initiated through action on central AhR ligands and downstream biotransformation and detoxification processes (
Madison et al. 2015,
2017;
Alsaadi et al. 2018). Though information on the PACs derived from OSPW is limited, levels of PACs present in OSPW have been shown to cause carcinogenic, mutagenic, immunotoxic, and endocrine disruptive effects in a variety of organisms (
Li et al. 2017). For example, Japanese medaka (
Oryzias latipes) exposed to OSPW water fractions had an inhibited ATP-binding cassette (ABC transport proteins) (
Alharbi et al. 2016). This inhibition of membrane protein activity could impede natural biotransformation and detoxification of PACs, thereby increasing severity of the toxic effects of OSPW on developing fish from NAFC alone.
Our finding that the prevalence of cardiovascular abnormalities was reduced with increased photocatalytic treatment is significant considering that
Leshuk et al. (2018b) observed that O
2−, OS
+, and NO
+ ions were rapidly transformed in early stages of photocatalytic treatment; further details of this treatment process on the chemical properties of NAs have been described previously (
de Oliveira Livera et al. 2018). As such, degradation of NAFC in OSPW may lower chronic toxicity by transforming the smaller toxic O
2 species, the predominant class of “classical” NAs oxygenated compounds in OSPW (
Barrow et al. 2014). Our data show that photocatalytic degradation of OSPW-derived NAs reduced the relative proportions of these lower molecular weight O
2 classes, but also decreased the proportion of higher molecular weight O
4–O
6 compounds while also increasing the diversity of these compounds (
Fig. 2).
Marentette et al. (2017) found that walleye embryos exposed to NAFC caused down-regulation of antioxidant defenses, which may also exacerbate the effects of oxidative stress. These O
2 classes appear to be a driver of the cardiovascular abnormalities as they may inhibit membrane transport proteins and damage DNA through oxidative stress.
Incomplete degradation of NAFC extracts by high-intensity treatment, e.g., >3 mg/L, elevated the incidence of cardiac abnormalities relative to untreated NAFC in the semi-natural experiment. This may be due to potential oxidized intermediates produced during the photocatalytic treatment, which could be more toxic than the result (
Leshuk et al. 2018b). This threshold is important when considering the practical application of buoyant TiO
2 microparticles to the tailings ponds in the oil sands region. Not only was more than 90% degradation via high-intensity treatment needed to reduce chronic toxicity to developing FHM in the laboratory, but incomplete degradation in lower treatment intensities may have the potential to enhance toxicity through these intermediates. Degradation of these specific compounds using similar photocatalytic conditions to those used in this experiment may best serve as a viable approach in tandem with more common biodegradative techniques. In related work,
de Oliveira Livera et al. (2018) demonstrated significant reduction in larger DBE compounds linked to acute narcosis in biological organisms using TiO
2-mediated photocatalysis of commercial mixtures used as analogs for OSPW-sourced NAs. However, its target effectiveness appears to be related more to the ability to degrade toxic intermediates made up of larger DBE compounds into smaller O
x species. Here, the reduction of extracted NAFC toxicity indicated by the reduction of NA-O
2 concentrations under 3 mg/L using this method of photocatalysis appears necessary to meet intended targets for both acute and chronic toxicity. However, should any additional toxic intermediates remain in these fractions they may require additional biotransformation for full remediation of those compounds with suspected longer-term effects on aquatic organisms. For example, earlier studies have also demonstrated the longer-term impact of OSPW toxicity on endocrine function in FHM at similar and environmentally relevant concentrations of oilsands NAs from OSPW (
Kavanagh et al. 2012,
2013). Whether these types of effects are alleviated by the photocatalytic treatment remains unexamined at this time.
Dissimilarity in larval mortalities and malformations at hatch were also observed between treatment groups (Supplementary
Fig. S1). However, when these fish were moved to clean water following the exposure, no difference in growth was noted 7 d after the exposure to NAFC treatment solutions. Post-hatch mortality in embryos exposed to low-intensity treated NAFC may be related to the cardiac abnormalities observed with aforementioned change in proportions of oxygen-containing compounds in NAFC extracts following oxidative treatment. The cardiac abnormalities in larval FHM may impair available energy for important developmental processes or osmoregulatory abilities, resulting in higher post-hatch mortality. This finding is consistent with a previous study that found zebrafish with pericardial edemas caused by embryonic exposure to crude oil generally failed to feed as larvae and died before the onset of free feeding (
Hicken et al. 2011). Though embryos were able to survive to hatch in the embryotoxicity bioassay, exposure to NAFC extracts may have lasting effects on FHM performance and survival later in life. These findings together emphasize the need to further explore the effectiveness of photocatalysis to treat both immediate and long-term toxic effects stemming from NAs and related compounds extracted from oil sands effluents.
The photocatalytic treatment of NAFC extracts was by and large successful at reducing the overall toxicity of OSPW-derived NAs to developing fish, but our study demonstrates the harmful consequences of incomplete degradation of NAs to aquatic vertebrates. The power of “green” (i.e., sustainable, recyclable, and reusable) buoyant microparticles over TiO
2, along with an unlimited solar energy source, makes implementing this type of passive degradation method an attractive technology for large-scale OSPW treatment in the oil sands industry. Future work in this area would benefit from more specific chemical characterization of NAFC from OSPW (e.g., atmospheric-pressure photo-ionization-HRMS, liquid chromatography or gas chromatography, or tandem MS) to streamline advanced oxidative processes and target catalytic degradation treatments to desired NAs that drive toxicity in aquatic animals. More broadly, we recommend that careful consideration should be paid not only to the feasibility and energy demands of any advanced oxidation treatments (
McQueen et al. 2016), but as we demonstrate here, rigorous testing and monitoring must be performed to prevent the unintended ecological consequences caused by incomplete degradation of NAs and other fraction components in discharged waters.