Indigenous-led camera-trap research on traditional territories informs conservation decisions for resource extraction
Abstract
The resource extraction that powers global economies is often manifested in Indigenous Peoples’ territories. Indigenous Peoples living on the land are careful observers of resulting biodiversity changes, and Indigenous-led research can provide evidence to inform conservation decisions. In the Nearctic western boreal forest, landscape change from forest harvesting and petroleum extraction is intensive and extensive. A First Nations community in the Canadian oil sands co-created camera-trap research to explore observations of presumptive species declines, seeking to identify the relative contributions of different industrial sectors to changes in mammal distributions. Camera data were analyzed via generalized linear models in a model-selection approach. Multiple forestry and petroleum extraction features positively and negatively affected boreal mammal species. Pipelines had the greatest negative effect size (for wolves), whereas well sites had a large positive effect size for multiple species, suggesting the energy sector as a target for co-management. Co-created research reveals spatial relationships of disturbance, prey, and predators on Indigenous traditional territories. It provides hypotheses, tests, and interpretations unique to outside perspectives; Indigenous participation in conservation management of their territories scales up to benefit global biodiversity conservation.
Graphical Abstract
Introduction
The agricultural and industrial revolutions that followed European colonization led to widespread landscape change and ushered the Anthropocene era, in which global systems are largely human dominated (Vitousek et al. 1997; Steffen et al. 2011; Maxwell et al. 2016). The resource extraction that powers global economies is often manifested in Indigenous Peoples’ traditional territories (Veltmeyer 2013; Gilberthorpe and Hilson 2016). However Indigenous Peoples are often excluded from conservation decisions, and their traditional ecological knowledge is often omitted from decision-making (Thompson et al. 2020). Global economies depend on development across thousands of traditional territories worldwide; empowering Indigenous communities to manage development on their territories will scale up for positive impacts on global biodiversity (Garnett et al. 2018).
Across North America, mammalian species have declined markedly since European colonization (Laliberte and Ripple 2004). However, even in the “remote” regions of the boreal Nearctic, human occupation dates back millennia (Goebel et al. 2008). First Nations coexisted with wildlife and in some places managed the land using methods such as prescribed burning (Kimmerer and Lake 2001), and Indigenous land practices have led to increased forest biodiversity (Fisher et al. 2019). In many areas Indigenous lands’ biodiversity equals that of federally defined and managed protected areas (Schuster et al. 2019).
Indigenous Peoples have a strong tradition of oral history, so they are uniquely positioned to observe and interpret changing conditions within the context of recent history (Gilchrist et al. 2005; Kendrick and Manseau 2008; Parlee et al. 2014; Kohler et al. 2019). However, consultation for resource extraction projects is often cursory and superficial (Baker and Westman 2018) and Indigenous knowledge often discounted. Partnering Indigenous knowledge and western science holds great promise for understanding how systems work and how anthropogenic change affects those systems (Mistry and Berardi 2016; Popp et al. 2019), provided it overcomes many noted social barriers (Carman et al. 2019). Here, we illustrate how Indigenous-led research guided by traditional ecological knowledge can identify the relationships between industrial landscape features and mammal distribution, to inform better conservation decisions in areas of dramatic change.
The western Nearctic boreal forest has remained more or less intact until the late 20th century, when widespread forest harvesting caused changes to mammal (Fisher and Wilkinson 2005) and bird (Schieck and Song 2006) communities. Soon thereafter, petroleum extraction proved economically viable and spread across the western boreal forest, creating widespread disturbances unique in their shapes and in their age and size distributions (Pickell et al. 2013; Pickell et al. 2015). Resource extraction has impacted mammal distributions (Fisher and Burton 2018) and populations (Burgar et al. 2019). Pressingly, landscape change has generated precipitous woodland caribou (Rangifer tarandus) declines (Boutin et al. 2012). Within this landscape, which supports substantial North American resource extraction economies, live two dozen Indigenous communities who experience first-hand the environmental and socioeconomic effects of development stemming from ineffective government consultation and alienation from decision-making, such that that many feel resigned to suffer the loss of their land and its biodiversity (Westman and Joly 2019).
Within this landscape, the co-authors—Indigenous Peoples of the Whitefish Lake First Nation (WLFN), a Treaty 8 (Fumoleau 2004) Nation of 3000 people—have a long tradition of subsistence hunting and close ties with the land. This long relationship has provided the WLFN co-authors with traditional ecological knowledge of the land and its species; knowledge collected has been passed down inherently through generations. It has been ingrained into a way of thinking naturally. Recently the co-authors observed changes to the wildlife populations on their land: Wherever there are forest harvesting cutblocks, wolves (Canis lupus) multiply and moose (Alces alces) and white-tailed deer (Odocoileus virginianus) decline. This varies with water and snow availability in each season. When there is deeper snow, wolf travel becomes harder, and more moose survive. In summer, if there is a lot of water, moose survive in wet areas. In dry seasons with forest fires, moose move from their typical habitats and the wet habitat is lost to them. Fewer moose become available for First Nations subsistence hunting. In the northeast of the territory, a large forest fire burned in 2011. The fire left more dead trees on the ground, providing cover as the dead trees fall over. Snowshoe hares (Lepus americanus) have higher tendency to survive due to the cover provided from fallen trees, which provide protection from predators. Lynx (Lynx canadensis) follow those hares, and so do coyote (Canis latrans), so both are more common in those disturbed areas. The increase in industrial activities has also led to fewer moose. Fewer moose are observed in roaded areas due to collisions and exposure to poaching (personal observation).
Declines in moose food sources are particularly troubling. Forest harvesting usually increases moose forage and hence relative abundance, but may exclude moose where patch sizes and context are insufficient for protection cover and other life-history requirements (Fisher and Wilkinson 2005). The Nation’s knowledge suggests this latter case, with moose faring poorly in cutblocks and other cleared landscape features. Moreover, a common prey of wolves, coyotes, and lynx—snowshoe hare—increases in disturbed areas, with subsequent increases in these predators.
With traditional ecological knowledge suggesting changes to mammal populations in response to resource extraction, there is impetus to identify the landscape features and associated industrial sectors affecting mammal distribution as targets for conservation. Forestry and petroleum sectors are managed separately, and each generates different landscape features. Energy exploration creates linear “seismic lines” (Dabros et al. 2018); energy extraction creates oil wells, pipelines, and processing facilities; forest harvesting creates polygonal forest harvest blocks; and the transportation sector creates networks of roads and trails to support these activities. Cumulatively these induce marked biotic change on mammal communities (Fisher and Burton 2018; Wittische et al. 2021), but evidence for which sector has the biggest influence remains unknown. Identifying the landscape features and associated industry responsible for change allows the Nation to funnel consultation resources into discussion with that sector and is the first step to targeted conservation (Westman and Joly 2019).
We used camera trapping (Burton et al. 2015; Steenweg et al. 2017) to identify which landscape features best explained variability in mammal distribution across the territory. Traditional Knowledge held by First Nations is made of long-term observations, but we do not have companion western science data on population trends, so we are constrained to contemporary data. However, if anthropogenic landscape features are avoided by a species, and associated with lower space-use across the territory, we made the logical assumption that these measures are evidentiary of potential population declines (Hui et al. 2009; Clare et al. 2015; Linden et al. 2017). Our goal was to weigh the relative importance of anthropogenic features—such as petroleum exploration seismic lines (Dickie et al. 2017; Dabros et al. 2018; Serrouya et al. 2020), forest harvest cutblocks (Fisher and Wilkinson 2005), and well sites, industrial sites, roads, and trails (Fisher and Burton 2018)—in explaining spatial variability in boreal mammal species’ distributions. Using camera-trapping data, generalized linear models, and Indigenous knowledge, we sought to understand the extent to which different kinds of features—those offering resource subsidies, or movement subsidies, or habitat loss (Fisher and Burton 2018)—affect mammal species in this complex landscape.
Methods
Study area
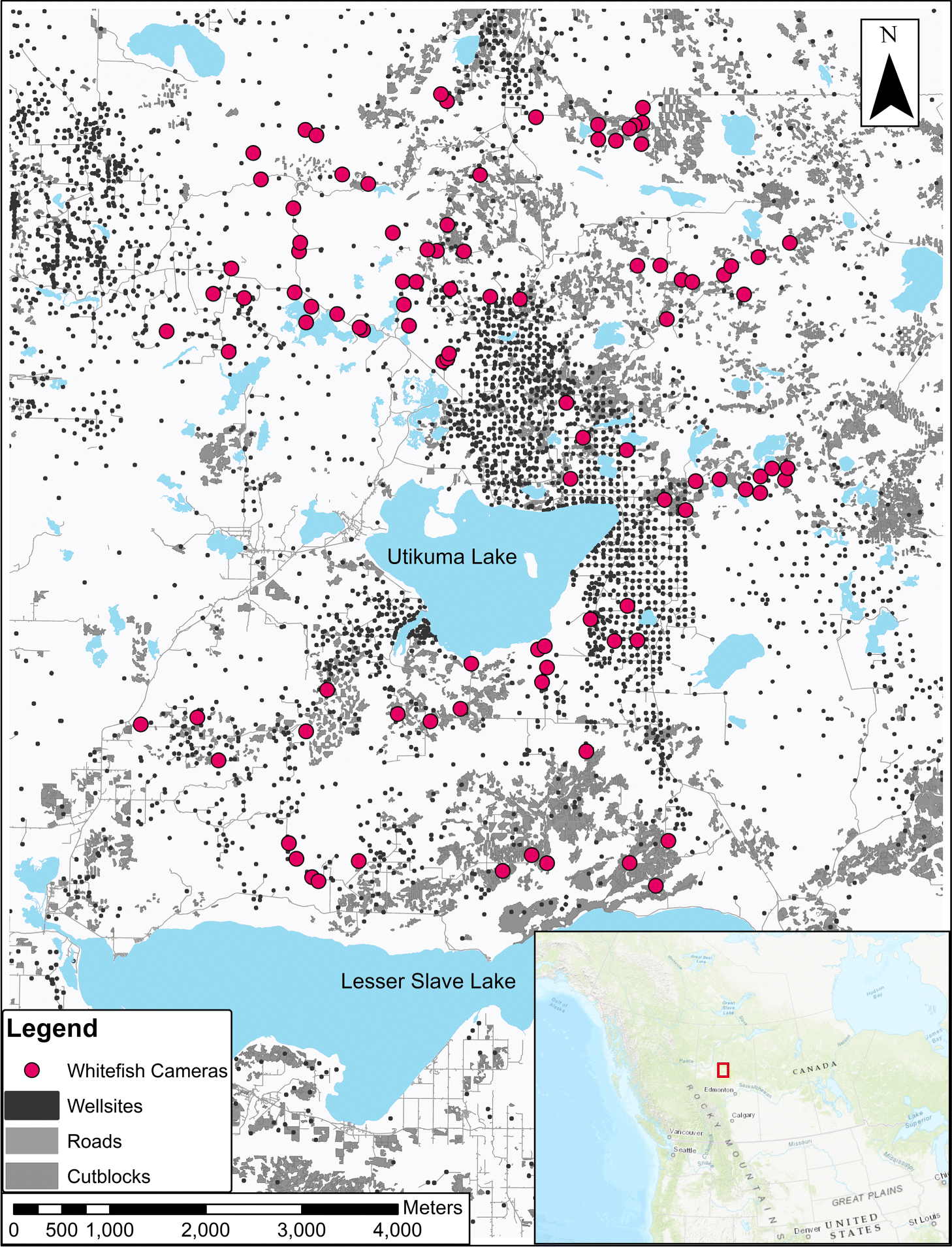
We surveyed mammal distribution on the WLFN Treaty 8 Territory in north-central Alberta, Canada (Fig. 1), a heterogeneous mosaic of white spruce (Picea glauca), black spruce (Picea mariana), aspen (Populus tremuloides), and Ledum groenlandicum-dominated muskeg. Petroleum exploration and extraction features, roads, forest harvesting, and off-road trails are dispersed throughout (Fig. 1).
Fig. 1.
Study design
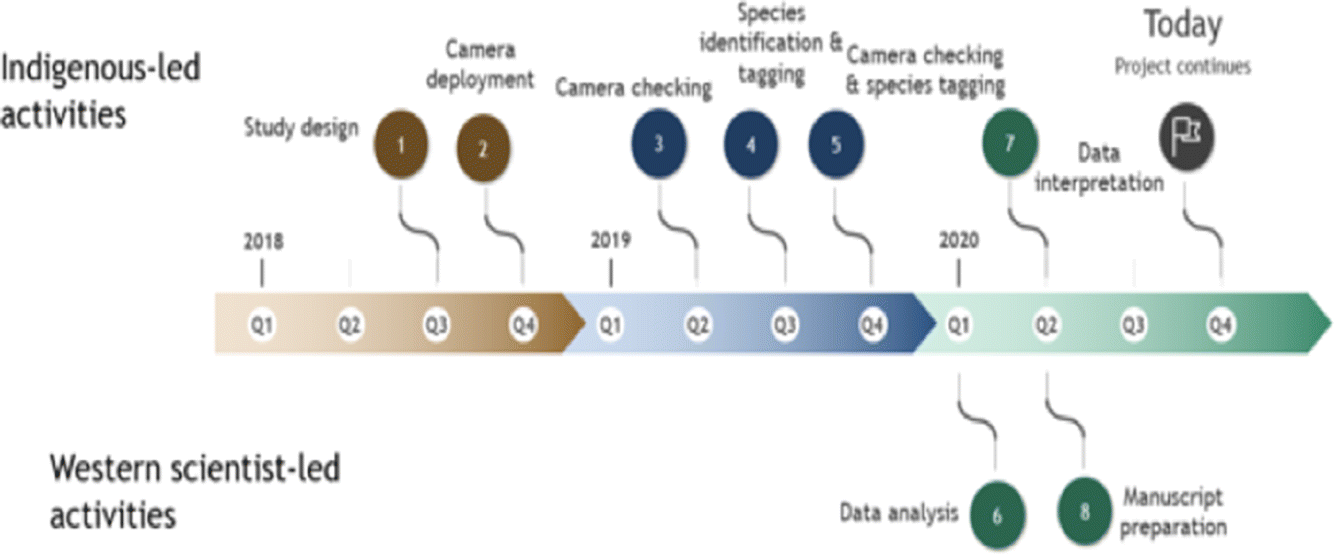
Through 2018–2019 WLFN people and scientists discussed ecological knowledge about landscape changes and the design of camera-trapping research. Camera traps can serve as extra eyes on the landscape, staying out for long periods and recording mammals in remote locations, but designs must be tailored to ecological questions (Burton et al. 2015). We worked together (Fig. 2) to select a stratified sampling design covering a gradient of low–high disturbance, as well as the range of natural variation in forest cover, across the Territory. In a geographic information system (GIS) we reclassified Alberta Vegetation Index (AVI) forest inventory data into six landcover strata and randomly allocated sampling sites equally to each; this design minimized collinearity among landscape feature variables, while distributing sampling effort across gradients of development and natural variability. We constrained sites to a minimum of 2 km and maximum of 4 km apart (Fig. 1) to satisfy requirements of future spatial capture-recapture models (Burgar et al. 2019; Royle et al. 2014).
Fig. 2.
Camera trapping
WLFN members deployed one ReconyxTM Hyperfire 2 camera (Holmen, WI, USA) at each site, set on active wildlife trails to increase probability of detecting an animal if present in the immediate vicinity (Stewart et al. 2019). A first set of 75 cameras were deployed December 2018 – April/May 2019. An additional 25 cameras provided a full set of 100 cameras active March – November 2019. Cameras were placed approximately 1.5 m above ground on the bole of a tree facing the trail; camera sensors detect heat-in-motion and were set to “high sensitivity” to record one image with each motion detection, with no programmed delays between photographs. Images were identified to species by WLFN peoples using TimeLapse2 Imagine Analysis software.
Landcover data
Dominant natural landcover types were quantified from AVI, and anthropogenic features were quantified from the Alberta Biodiversity Monitoring Institute’s (ABMI) 2016 human footprint layer (HF), which yielded 89 landcover categories. We excluded categories with <0.5% coverage and combined remaining categories into 18 composite variables that represented the most relevant landscape features (Table S2).
In ArcGIS we drew 1000-m radius buffers around each camera site (Fisher et al. 2011) and calculated the percent area of each variable within those buffers. To enable comparison of effect sizes we scaled all landscape variables (mean = 0, SD = 1). We explored collinearity and conducted variance inflation estimation (Zuur et al. 2007; Zuur et al. 2010), and confirmed there was no collinearity (VIF < 3) among independent variables.
Model structure
Camera-trap data were discretized monthly; each month is considered an independent Bernoulli trial (Faraway 2016) in which a species was detected (1) or not (0) by the camera. We call this response variable “occurrence frequency”; it is the number of months of each species’ detections and nondetections, totaling the number of months sampled at that site. For example, a moose detected 5 months of a year would yield (5)(7), a proportional binomial.
To evaluate how each species’ occurrence frequency varies with land cover and anthropogenic features we applied generalized linear models (GLMs; binomial errors, log link) in R (R Core Development Team 2013) in a structured hypothesis framework. We modelled species’ monthly occurrence frequency as a function of natural land cover features plus anthropogenic variables, grouped into the following industry sectors: mining, oil extraction, oil exploration, forestry, and transportation (Table 1).
Table 1.
| Model set | Model no. | Variables | Feature description | Predicted association with species’ habitat use |
|---|---|---|---|---|
| Core natural | 1 | Deciduous + Mixedwood + Coniferous + Water + Meadow + Open Wetland + Aspen/Treed Wetland | Natural land cover classes | ± |
| Mining | 2 | Core natural model set + Borrow pits + Gravel pits | Natural land cover classes and block disturbance features associated with mining | − |
| Oil extraction | 3 | Core natural model set + Well sites + Pipelines | Natural land cover classes and both block and linear disturbance features associated with oil extraction | ± |
| Oil exploration | 4 | Core natural model set + Seismic lines + 3-D seismic lines | Natural land cover classes and linear features associated with oil exploration | ± |
| Forestry | 5 | Core natural model set + Young cutblocks + Old cutblocks | Natural land cover classes and block feature associated with forestry | + |
| Transportation | 6 | Core natural model set + Paved roads + Unpaved roads | Natural land cover classes and linear features associated with transportation | − |
| Null model | 7 | Intercept only | No features modelled | NA |
Our core model (1) included only natural land cover variables and represented our first hypothesis that species’ occurrence is best predicted by natural features (Table 1; Supplementary Material 1). Subsequent models added effects of industry to this core model. We predicted species to avoid mining features due to association with human activity and disturbance (model 2); however, we predicted both avoidance and selection of landscape features associated with oil extraction and oil exploration across boreal species. Oil extraction (model 3) was predicted to favor both predator and prey species due to provision of early seral stage resource subsidies. Oil exploration (model 4) creates seismic lines; we predicted carnivore species would select for these linear features as a travel corridor (Dickie et al. 2017) and ungulate species would avoid these features due to predation risk. Forestry (model 5) is expected to be positively associated with most species (Fisher and Wilkinson 2005) due to resources associated with early seral growth. We predicted species to generally avoid transportation features (model 6) due to hunting pressures and human disturbance. Our null hypothesis was represented by an intercept-only model (model 7).
Models were ranked using Akaike Information Criterion scores corrected for small sample size (AICc) (Burnham and Anderson 2002), balancing deviance explained and the number of model parameters. We calculated normalized AICc weights (Anderson 2007) that rank the relative support of the set of models between 0–1, analogous to the probability that the model is best in the candidate set. We interpreted the models with the highest AICc weights as the best-supported model describing boreal species distribution on the landscape and support for their corresponding hypotheses. We calculated evidence ratios (ER; Anderson 2007) to weigh the support of inclusion of industry covariates in the top-selected model against the core model containing only natural landcover variables. For example, ER = 2 indicates there is twice the evidence for inclusion of industry covariates than for exclusion. We assessed model fit and residual dispersion using diagnostic plots and calculated deviance explained for each model.
Results
We detected multiple mammal species across the Territory (Fig. 3). Snowshoe hare, moose, white-tailed deer, lynx, black bear (Ursus americanus), wolf, and coyote were most frequently detected (Table S2).
Fig. 3.

Species’ response to natural and anthropogenic land cover variables
Anthropogenic landscape features affected the distribution of all seven mammal species we examined. The best-supported models explaining species’ monthly occurrence included feature types associated with industry (Table 2).
Table 2.
| ΔAICc Score | |||||||
|---|---|---|---|---|---|---|---|
| Model set (#) | Moose | Snowshoe hare | Wolf | Lynx | Coyote | White-tailed deer | Black bear |
| Natural features (1) | 3.83 | 35.50 | 39.35 | 19.45 | 23.35 | 69.42 | 25.26 |
| Mining features (2) | 1.27 | 27.02 | 41.77 | 17.18 | 2.40 | 51.03 | 23.79 |
| Oil extraction (3) | 1.84 | 0.00 | 0.00 | 0.00 | 0.00 | 26.98 | 0.00 |
| Oil exploration (4) | 8.65 | 14.55 | 34.40 | 9.83 | 20.25 | 17.28 | 19.89 |
| Forestry (5) | 2.90 | 12.47 | 36.36 | 4.71 | 4.25 | 0.00 | 22.59 |
| Transportation (6) | 0.00 | 34.98 | 34.26 | 19.55 | 8.78 | 28.92 | 3.09 |
| Null (7) | 1.80 | 145.47 | 42.87 | 66.93 | 44.17 | 142.81 | 57.51 |
| ER: | 7.4 | >100 | >100 | >100 | >100 | >100 | >100 |
Note
The lowest ΔAICc score (bold underlined) represents the best-supported model for each species; bolded scores represent the next closest model. Evidence Ratios (ER) divide the AICc weight of the top model by that of the natural features model (Model 1).
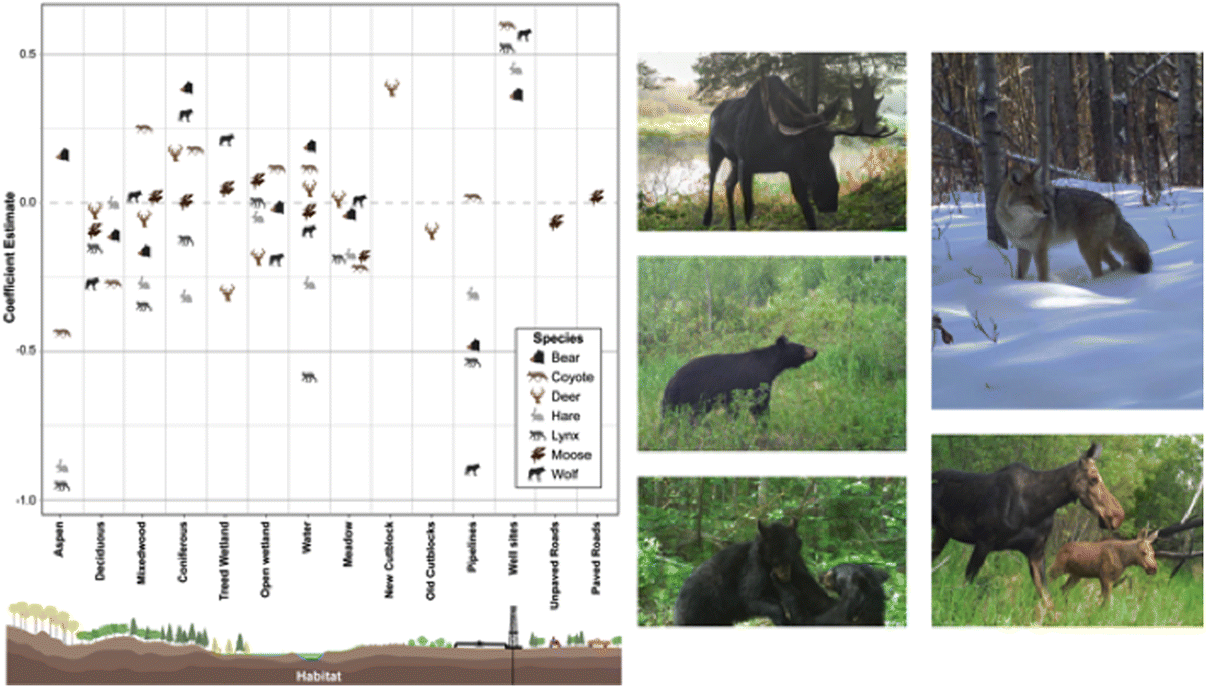
Black bear, wolf, coyote, lynx, and snowshoe hare occurrence were predominantly influenced by oil extraction features, specifically well sites and pipelines (Table 2; Fig. 4). Moose occurrence was best explained by transportation features and mining features. White-tailed deer occurrence was best explained by forestry features. With the exception of moose, the additive effects of industrial features markedly improved models’ explanatory power, compared with natural landcover alone (ER > 100; Table 2). Inclusion of transportation features (i.e., roads) improved our ability to predict moose occurrence over 7-fold (Table 2).
Fig. 4.
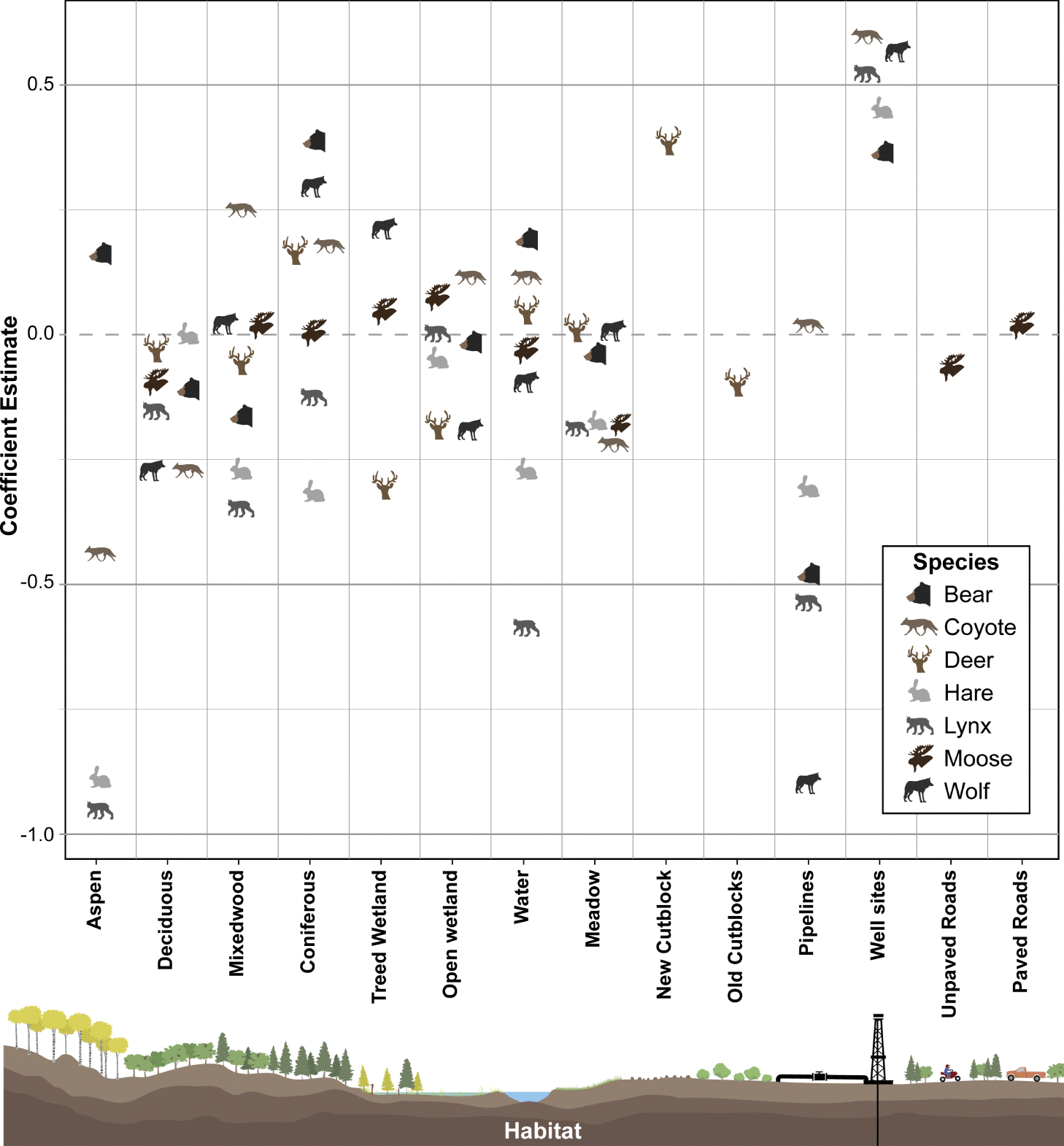
Petroleum extraction influenced the greatest number of species but the patterns of selection and avoidance differed among species (Fig. 4). Wolves, lynx, black bear, and snowshoe hare avoided areas with greater proportion of pipelines, whereas coyotes selected them. All of these species strongly selected areas with greater well-site density, with positive effect sizes greater than most natural features.
The role of natural versus anthropogenic landscape features in explaining moose occurrence is diverse (Table 2). Model support is insufficient to parse apart the explanatory power of transportation features from mining features (ΔAICc = 1.27; Table 2), or oil extraction features (ΔAICc = 1.84), or even the null model (ΔAICc = 1.80). The forestry model is also closely supported (ΔAICc = 2.9), so all these features may play a role, albeit with small effect sizes (Fig. 5). In summary, there is some evidence moose decrease with unpaved roads and increase with paved roads, decrease with new cutblocks and increase with old cutblocks, but the greatest effect sizes for moose were treed and open wetlands.
Fig. 5.
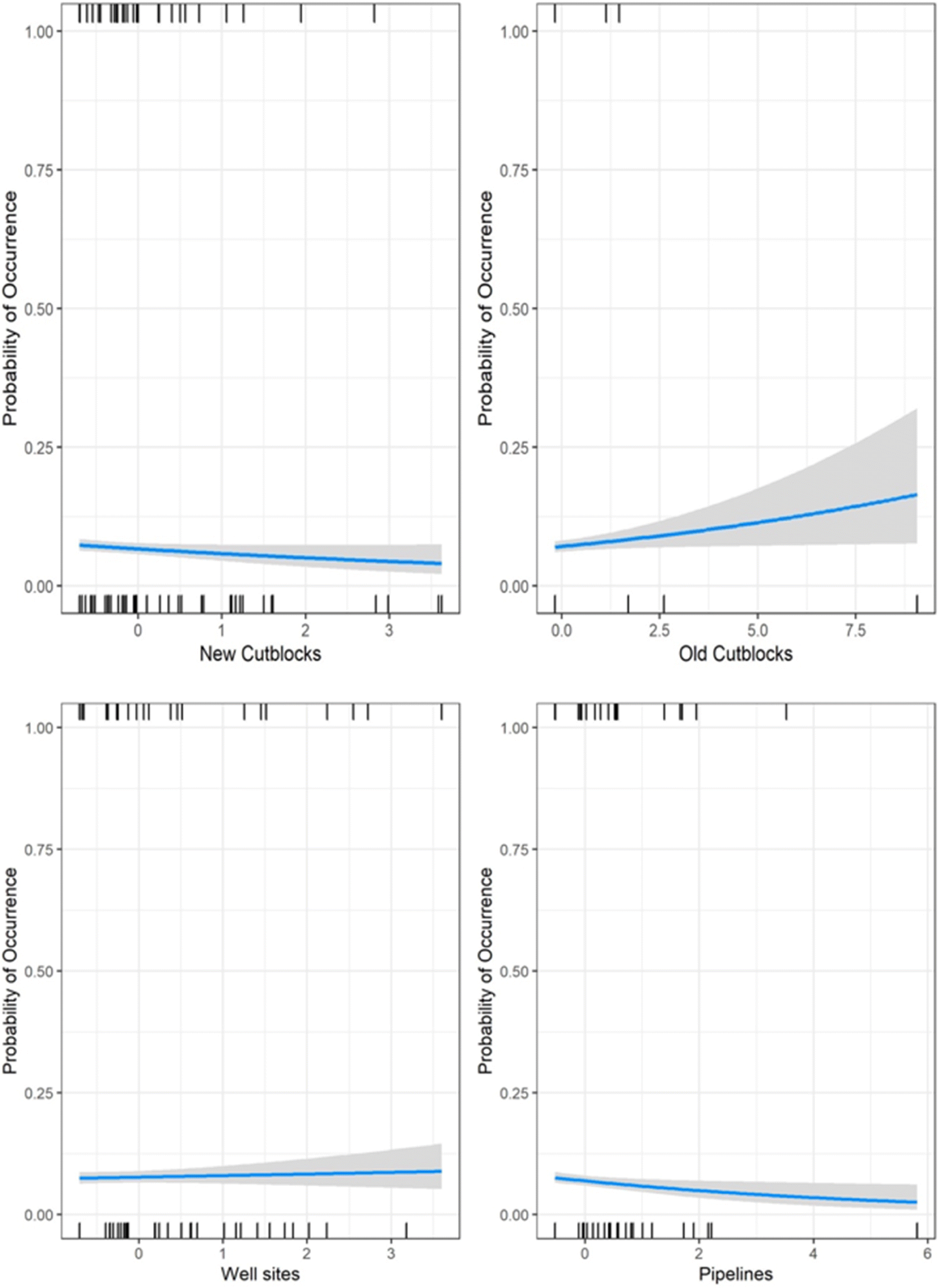
In contrast, white-tailed deer exhibited a very strong positive association with new cutblocks, with an effect size greater than any natural feature. We observed a weak avoidance of old cutblock stands in deer.
In summary, petroleum extraction features—oil well sites and pipelines—best explained distributions of five of the seven mammal species examined, generally outweighing the selection for any natural landcover variable. Forestry and transportation features explained some variability for deer and moose.
Discussion
Boreal forest mammals on the First Nation’s traditional territories are affected by the petroleum, forestry, and transportation sectors. The Canadian boreal forest landscape provides economic benefits of global importance (Bayoumi and Mühleisen 2006; Giesy et al. 2010; Heyes et al. 2018) but with costs absorbed by First Nations; although they live on these resource landscapes, they are rarely involved in management (Wellstead and Stedman 2008; Parlee 2015; Baker and Westman 2018; Westman and Joly 2019).
Illustrating cumulative effects of multiple sectors has been a Western science goal (Fisher and Burton 2018; Heim et al. 2017), but identifying the landscape features and associated sectors most responsible for biotic change is a key starting point for First Nations wildlife co-management (Baker and Westman 2018; Westman and Joly 2019). Co-created research informed by traditional ecological knowledge (Abu et al. 2019; Colbourne et al. 2019; Skroblin et al. 2019; Wheeler et al. 2020) provides insights important to effective conservation. Here, the WLFN coauthors’ traditional ecological knowledge informed a series of model hypotheses that help identify landscape features associated with mammal distributions and identified the specific landscape feature(s) causing change among multiple species. Although co-authors suggested forest harvesting was a primary sector affecting wildlife, this camera-data research suggests oil and gas is generating the most change. We discovered that changes among predators and prey can be traced to changes in the density of well sites on the landscape, with a contribution of forest harvesting through inflated white-tailed deer, which are invasive on this landscape (Fisher and Burton 2020; Fisher et al. 2020). Although these changes were expected to be contextual with recent fire, we did not parse this apart with our industry-focused design.
Moose are a particularly important species here and WLFN traditional ecological knowledge indicates moose were less abundant in places with forestry and energy extraction on the traditional territory. This knowledge corroborates evidence from camera-traps and statistical models; the influence of several sectors is apparent. The Nation observed that oil wells disturb habitats and human activity on associated roads and trails—by energy workers and hunters—make these areas unsuitable habitat. The Nation also identified that new cutblocks on their lands are unable to sustain moose; although this was not the strongest driver of moose occurrence, it was nonetheless detectable. Armed with this evidence, better decisions about land management to conserve moose can be made—provided Indigenous voices are heard.
Indigenous-led research also unveils hidden complexities. Oil and gas well sites are pervasive on the western boreal landscape and they generate spatial patterns completely unique from natural disturbance (Pickell et al. 2013; Pickell et al. 2015). Well site deployment removes mature forest and replaces it with early seral vegetation. However, well sites are on average 1 ha in size (smaller than forest harvest blocks and fires) and are pervasive (Fig. 1), creating hundreds of patches of young vegetation embedded within an otherwise intact forested matrix. Well sites offer a resource subsidy that snowshoe hares capitalized upon, likely bolstered by the 2011 forest fire as per the WLFN co-authors’ observations. Also following their observations, hare predators—lynx, coyote, and wolf—then exploit this abundance of hares. Lynx, as snowshoe hare specialists (Ward and Krebs 1985; Murray et al. 1994; Stenseth et al. 1997), cue into these prey-rich sites. Coyotes, which prey upon snowshoe hares (Murray et al. 1994; O’Donoghue et al. 1997; O’Donoghue et al. 1998a, 1998b) and other small and mid-sized mammals (Dumond et al. 2001), likewise select well pads with their abundant prey. Wolves prey on these same small mammals (Floyd et al. 1978; Thurber et al. 1992; Weaver 1993; Arjo et al. 2002; Urton and Hobson 2005), and our data suggest wolves too are cueing in on well sites. Black bears use both vegetation and prey subsidies. Thus, the pattern the WLFN co-authors observed with fires—of hares increasing followed by predators—is also mirrored in areas with high well site density.
Wolves (Bergerud et al. 1983; Messier and Crête 1985; Marshal and Boutin 1999) and coyotes (Benson and Patterson 2013) prey on moose. Thus, with increased small prey use comes increased predator density, and well sites—which would otherwise offer an early-seral vegetation resource subsidy, but instead represent a high-risk landscape feature for moose, which avoid them. Indigenous knowledge corroborates this contention: the Nation has repeatedly observed that moose use developed areas for only 2–3 d then move on. Wolves follow them into these areas, pushing them off, or killing them. Given that well sites offer early seral vegetation resource subsidies embedded in escape cover, and that comparable small forest harvest areas are elsewhere selected by moose (Fisher and Wilkinson 2005), the predator avoidance hypothesis appears to offer a parsimonious explanation and is supported both by Indigenous ecological knowledge and by evidence from camera-trap data.
Past western science suggests linear features are key to wolves, as linear features expedite travel (Dickie et al. 2017) especially in winter, thereby increasing predation rates on woodland caribou (Whittington et al. 2011; McKenzie et al. 2012). In this landscape, effects of seismic lines did not predominate, and instead well sites best explained wolf distribution. Although seismic lines are the focus for landscape restoration efforts in western Canada (Dabros et al. 2018; Tattersall et al. 2019; Serrouya et al. 2020), this Indigenous-led research suggests well sites should also be a focus of restoration. Co-management of landscape planning between industry and the Nation might prevent negative effects on wildlife from the start.
Conclusions
Our approach identifies the most important targets for management on traditional territories, most likely to produce positive change. Though landscape management for conservation across the whole boreal may be prohibitively expensive, targeting the foremost problem for the most species is a wise approach for triage (Schneider et al. 2010; Hebblewhite 2017).
Indigenous-led research can be used as globally as the technology allows (Steenweg et al. 2017) to inform statistical models built from Indigenous knowledge (Skroblin et al. 2019), and resulting conservation decisions can yield substantial positive outcomes for conservation science (Carman et al. 2019; Kohler et al. 2019). Co-management of resources is an evolving trend globally (Castro and Nielsen 2001; Spak 2005; Ross et al. 2009), hopefully replacing consultation processes that are ineffective in implementing vital conservation knowledge and advice (Thompson et al. 2020).
The United Nations Declaration on the Rights of Indigenous Peoples (Gilbert 2007) calls for First Nations’ involvement in development of their lands. Consultation processes are often insufficient for conservation (Baker and Westman 2018; Westman and Joly 2019; Thompson et al. 2020) as witnessed by continuing biodiversity declines in extraction landscapes around the world. We illustrate how co-created Indigenous-led research that informs the conceptual foundation for research, the goals, the hypotheses, and the interpretation of results, yields valuable insights different to a single western-science perspective: in this case, multiple wildlife species are more likely to occur in areas with dense petroleum well sites. Multiple modes of knowledge are needed to tease apart the growing complexity of species interactions and response to disturbance in rapidly changing landscapes, hopefully leading to better management decisions to conserve biodiversity.
Acknowledgements
We are thankful to Environment and Climate Change Canada for funding this work. Thanks to the Elders of Whitefish Lake First Nation for their teaching and guidance to the co-authors. Thanks to Alina Fisher for manuscript review and Jeff Dixon for illustrations (Fig. 4). Finally, we are thankful to Brian Tallman, Band Administrator at Whitefish Lake First Nation, and Chief and Council for facilitating us.
References
Abu R, Reed MG, and Jardine TD. 2019. Using two-eyed seeing to bridge Western science and Indigenous knowledge systems and understand long-term change in the Saskatchewan River Delta, Canada. International Journal of Water Resources Development, 36(5): 1–20.
Anderson DR. 2007. Model based inference in the life sciences: a primer on evidence. Springer Science & Business Media.
Arjo WM, Pletscher DH, and Ream RR 2002. Dietary overlap between wolves and coyotes in northwestern Montana. Journal of Mammalogy, 83(3): 754–766.
Baker JM, and Westman CN. 2018. Extracting knowledge: Social science, environmental impact assessment, and Indigenous consultation in the oil sands of Alberta, Canada. The Extractive Industries and Society, 5(1): 144–153.
Bayoumi T, and Mühleisen M. 2006. Energy, the exchange rate, and the economy: macroeconomic benefits of Canada’s oil sands production. No. 2006-2070. International Monetary Fund.
Benson J, and Patterson B. 2013. Moose (Alces alces) predation by eastern coyotes (Canis latrans) and eastern coyote × eastern wolf (Canis latrans × Canis lycaon) hybrids. Canadian Journal of Zoology, 91(11): 837–841.
Bergerud A, Wyett W, and Snider B. 1983. The role of wolf predation in limiting a moose population. The Journal of Wildlife Management, 47(4): 977–988.
Boutin S, Boyce MS, and Hebblewhite M. 2012. Why are caribou declining in the oil sands? Frontiers in Ecology and the Environment, 10(2): 65–67.
Burgar JM, Burton AC, and Fisher JT. 2019. The importance of considering multiple interacting species for conservation of species at risk. Conservation Biology, 33(3): 709–715.
Burnham K, and Anderson D. 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach 2nd EditionSpringer-Verlag. New York, New York.
Burton AC, Neilson, E., Moreira D, Ladle A, Steenweg R, Fisher JT, et al. 2015. Wildlife camera trapping: a review and recommendations for linking surveys to ecological processes. Journal of Applied Ecology, 52(3): 675–685.
Carman M, Carman VG, Cousseau MB, Pequeño G, Mabragaña E, Giussi A, et al. 2019. Going beyond diverse worldviews for conservation: response to Kohler et al. Conservation Biology, 34(1): 286–288.
Castro AP, and Nielsen E. 2001. Indigenous people and co-management: implications for conflict management. Environmental Science & Policy, 4(4–5): 229–239.
Clare JD, Anderson EM, and MacFarland, DM. 2015. Predicting bobcat abundance at a landscape scale and evaluating occupancy as a density index in central Wisconsin. The Journal of Wildlife Management, 79(3): 469–480.
Colbourne R, Moroz P, Hall, C, Lendsay, K, and Anderson RB. 2019. Indigenous works and two eyed seeing: mapping the case for indigenous-led research. Qualitative Research in Organizations and Management: An International Journal, 15(1): 68–86.
Dabros A, Pyper M, and Castilla G. 2018. Seismic lines in the boreal and arctic ecosystems of North America: environmental impacts, challenges, and opportunities. Environmental Reviews, 26(2): 214–229.
Dickie M, Serrouya R, McNay RS, and Boutin S. 2017. Faster and farther: wolf movement on linear features and implications for hunting behaviour. Journal of Applied Ecology, 54(1): 253–263.
Dumond M, Villard M-A, and Tremblay É. 2001. Does coyote diet vary seasonally between a protected and an unprotected forest landscape? Ecoscience, 8(3): 301–310.
Faraway JJ. 2016. Extending the linear model with R: generalized linear, mixed effects and nonparametric regression models. Chapman and Hall/CRC.
Fisher JA, Shackelford N, Hocking M.D, Trant AJ, and Starzomski BM. 2019. Indigenous peoples’ habitation history drives present-day forest biodiversity in British Columbia’s coastal temperate rainforest. People and Nature, 1(1): 103–114.
Fisher JT, and Wilkinson L. 2005. The response of mammals to forest fire and timber harvest in the North American boreal forest. Mammal Review, 35(1): 51–81.
Fisher JT, and Burton AC. 2018. Wildlife winners and losers in an oil sands landscape. Frontiers in Ecology and the Environment, 16(6): 323–328.
Fisher JT, and Burton AC. 2020. Spatial structure of reproductive success infers mechanisms of ungulate invasion in Nearctic boreal landscapes. Ecology and Evolution, 11(2): 900–911.
Fisher JT, Anholt B, and Volpe JP. 2011. Body mass explains characteristic scales of habitat selection in terrestrial mammals. Ecology and evolution, 1(4): 517–528.
Fisher JT, Burton AC, Nolan L, and Roy L. 2020. Influences of landscape change and winter severity on invasive ungulate persistence in the Nearctic boreal forest. Scientific Reports, 10(1): 1–11.
Floyd TJ, Mech LD, and Jordan PA. 1978. Relating wolf scat content to prey consumed. The Journal of Wildlife Management, 42(3): 528–532.
Fumoleau R. 2004. As long as this land shall last: A history of Treaty 8 and Treaty 11, University of Calgary Press. pp. 1870–1939.
Garnett ST, Burgess ND, Fa JE, Fernández-Llamazares Á, Molnár Z, Robinson CJ, et al. 2018. A spatial overview of the global importance of Indigenous lands for conservation. Nature Sustainability, 1(7): 369–374.
Giesy JP, Anderson JC, and Wiseman SB. 2010. Alberta oil sands development. Proceedings of the National Academy of Sciences, 107(3): 951–952.
Gilbert J. 2007. Indigenous rights in the making: The United Nations Declaration on the Rights of Indigenous Peoples. International Journal of Minority and Group Rights, 14: 207.
Gilberthorpe E, and Hilson G. 2016. Natural resource extraction and indigenous livelihoods: Development challenges in an era of globalization. Routledge.
Gilchrist G, Mallory M, and Merkel F. 2005. Can local ecological knowledge contribute to wildlife management? Case studies of migratory birds. Ecology and Society, 10(1): 12.
Goebel T, Waters MR, and O’Rourke DH. 2008. The late Pleistocene dispersal of modern humans in the Americas. Science, 319(5869): 1497–1502.
Hebblewhite M. 2017. Billion dollar boreal woodland caribou and the biodiversity impacts of the global oil and gas industry. Biological Conservation, 206: 102–111.
Heim N, Fisher JT, Clevenger A, Paczkowski J, and Volpe J. 2017. Cumulative effects of climate and landscape change drive spatial distribution of Rocky Mountain wolverine (Gulo gulo L.). Ecology and Evolution, 7(21): 8903–8914.
Heyes A, Leach A, and Mason CF. 2018. The economics of Canadian oil sands. Review of Environmental Economics and Policy, 12(2): 242–263.
Hui C, McGeoch MA, Reyers B, Roux PC, Greve M, and Chown SL. 2009. Extrapolating population size from the occupancy–abundance relationship and the scaling pattern of occupancy. Ecological Applications, 19(8): 2038–2048.
Kendrick A, and Manseau M. 2008. Representing traditional knowledge: Resource management and Inuit knowledge of barren-ground caribou. Society and Natural Resources, 21(5): 404–418.
Kimmerer RW, and Lake FK. 2001. The role of indigenous burning in land management. Journal of Forestry, 99(11): 36–41.
Kohler F, Holland TG, Kotiaho JS, Desrousseaux M, and Potts MD. 2019. Embracing diverse worldviews to share planet Earth. Conservation Biology, 33(5): 1014–1022.
Laliberte AS, and Ripple WJ. 2004. Range contractions of North American carnivores and ungulates. BioScience, 54(2): 123–138.
Linden DW, Fuller AK, Royle JA, and Hare MP. 2017. Examining the occupancy–density relationship for a low-density carnivore. Journal of Applied Ecology, 54(6): 2043–2052.
Marshal JP, and Boutin S. 1999. Power analysis of wolf-moose functional responses. The Journal of Wildlife Management, 63(1): 396–402.
Maxwell SL, Fuller RA, Brooks TM, and Watson JE. 2016. Biodiversity: The ravages of guns, nets and bulldozers. Nature News, 536(7615): 143.
McKenzie HW, Merrill EH, Spiteri RJ, and Lewis MA. 2012. How linear features alter predator movement and the functional response. Interface Focus, 2(2): 205–216.
Messier F, and Crête M. 1985. Moose-wolf dynamics and the natural regulation of moose populations. Oecologia, 65(4): 503–512.
Mistry J, and Berardi A. 2016. Bridging indigenous and scientific knowledge. Science, 352(6291): 1274–1275.
Murray DL, Boutin S, and O’Donoghue M. 1994. Winter habitat selection by lynx and coyotes in relation to snowshoe hare abundance. Canadian Journal of Zoology, 72(8): 1444–1451.
O’Donoghue M, Boutin S, Krebs CJ, and Hofer EJ. 1997. Numerical responses of coyotes and lynx to the snowshoe hare cycle. Oikos, 80(1): 150–162.
O’Donoghue M, Boutin, S, Krebs CJ, Murray DL, and Hofer EJ. 1998a. Behavioural responses of coyotes and lynx to the snowshoe hare cycle. Oikos, 82(1): 169–183.
O’Donoghue M, Boutin S, Krebs CJ, Zuleta G, Murray DL, and Hofer EJ. 1998b. Functional responses of coyotes and lynx to the snowshoe hare cycle. Ecology, 79(4): 1193–1208.
Parlee BL. 2015. Avoiding the resource curse: indigenous communities and Canada’s oil sands. World Development, 74: 425–436.
Parlee BL, Goddard E, First Nation ŁKéD, and Smith M. 2014. Tracking change: Traditional knowledge and monitoring of wildlife health in Northern Canada. Human Dimensions of Wildlife, 19(1): 47–61.
Pickell PD, Andison DW, and Coops NC. 2013. Characterizations of anthropogenic disturbance patterns in the mixedwood boreal forest of Alberta, Canada. Forest Ecology and Management, 304: 243–253.
Pickell PD, Andison DW, Coops NC, Gergel SE, and Marshall PL. 2015. The spatial patterns of anthropogenic disturbance in the Western Canadian boreal forest following oil and gas development. Canadian Journal of Forest Research, 45(6): 732–743.
Popp JN, Priadka P, and Kozmik C. 2019. The rise of moose co-management and integration of Indigenous knowledge. Human Dimensions of Wildlife, 24(2): 159–167.
R Core Development Team. 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Ross H, Grant C, Robinson CJ, Izurieta A, Smyth D, and Rist P. 2009. Co-management and Indigenous protected areas in Australia: achievements and ways forward. Australasian Journal of Environmental Management, 16(4): 242–252.
Royle JA, Chandler RB, Sollmann R, and Gardner B. 2014. Spatial capture-recapture. Academic Press.
Schieck J, and Song SJ. 2006. Changes in bird communities throughout succession following fire and harvest in boreal forests of western North America: literature review and meta-analyses. Canadian Journal of Forest Research, 36(5): 1299–1318.
Schneider RR, Hauer G, and Boutin S. 2010. Triage for conserving populations of threatened species: the case of woodland caribou in Alberta. Biological Conservation, 143(7): 1603–1611.
Schuster R, Germain RR, Bennett JR, Reo NJ, and Arcese P. 2019. Vertebrate biodiversity on indigenous-managed lands in Australia, Brazil, and Canada equals that in protected areas. Environmental Science & Policy, 101: 1–6.
Serrouya R, Dickie M. DeMars C, Wittmann M, and Boutin S. 2020. Predicting the effects of restoring linear features on woodland caribou populations. Ecological Modelling, 416: 108891.
Skroblin A, Carboon T, Bidu G, Chapman N, Miller M, Taylor K, et al. 2019. Including Indigenous knowledge in species distribution modelling for increased ecological insights. Conservation Biology, 35(2): 587–597.
Spak S. 2005. The position of indigenous knowledge in Canadian co-management organizations. Anthropologica, 47(2): 233–246.
Steenweg R, Hebblewhite M, Kays R, Ahumada J, Fisher JT, Burton C, et al. 2017. Scaling-up camera traps: Monitoring the planet’s biodiversity with networks of remote sensors. Frontiers in Ecology and the Environment, 15(1): 26–34.
Steffen W, Grinevald J, Crutzen P, and McNeill J. 2011. The Anthropocene: conceptual and historical perspectives. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 369(1938): 842–867.
Stenseth NC, Falck W, Bjørnstad ON, and Krebs CJ. 1997. Population regulation in snowshoe hare and Canadian lynx: asymmetric food web configurations between hare and lynx. Proceedings of the National Academy of Sciences, 94(10): 5147–5152.
Stewart FE, Volpe JP, and Fisher JT. 2019. The debate about bait: A red herring in wildlife research. The Journal of Wildlife Management, 83(4): 985–992.
Tattersall ER, Burgar JM, Fisher JT, and Burton AC. 2019. Mammal seismic line use varies with restoration: Applying habitat restoration to species at risk conservation in a working landscape. Biological Conservation, 108295.
Thompson K-L, Lantz T, and Ban N. 2020. A review of Indigenous knowledge and participation in environmental monitoring. Ecology and Society, 25(2).
Thurber JM, Peterson RO, Woolington JD, and Vucetich JA. 1992. Coyote coexistence with wolves on the Kenai Peninsula, Alaska. Canadian Journal of Zoology, 70(12): 2494–2498.
Urton EJ, and Hobson KA. 2005. Intrapopulation variation in gray wolf isotope (δ 15 N and δ 13 C) profiles: implications for the ecology of individuals. Oecologia, 145(2): 316–325.
Veltmeyer H. 2013. The political economy of natural resource extraction: a new model or extractive imperialism? Canadian Journal of Development Studies/Revue canadienne d'études du développement, 34(1): 79–95.
Vitousek PM, Mooney HA, Lubchenco J, and Melillo JM. 1997. Human domination of Earth’s ecosystems. Science, 277(5325): 494–499.
Ward RM, and Krebs CJ. 1985. Behavioural responses of lynx to declining snowshoe hare abundance. Canadian Journal of Zoology, 63(12): 2817–2824.
Weaver JL. 1993. Refining the equation for interpreting prey occurrence in gray wolf scats. The Journal of Wildlife Management, 534–538.
Wellstead A, and Stedman RC. 2008. Intersection and integration of First Nations in the Canadian forestry sector. Journal of Aboriginal Economic Development, 6(1): 30–43.
Westman CN, and Joly TL. 2019. Oil sands extraction in Alberta, Canada: A review of impacts and processes concerning Indigenous peoples. Human Ecology, 47(2): 233–243.
Wheeler H, Danielsen F, Fidel M, Hausner V, Horstkotte T, Johnson N, et al. 2020. The need for transformative changes in the use of Indigenous knowledge along with science for environmental decision-making in the Arctic. People and Nature, 2(3): 544–556.
Whittington J, Hebblewhite M, DeCesare NJ, Neufeld L, Bradley M, Wilmshurst J, and Musiani M. 2011. Caribou encounters with wolves increase near roads and trails: a time-to-event approach. Journal of Applied Ecology, 48(6): 1535–1542.
Wittische J, Heckbert S, James PMA, Burton AC, and Fisher JT. 2021. Community-level modelling of boreal forest mammal distribution in an oil sands landscape. Science of The Total Environment 755: 142500.
Zuur A, Ieno EN, and Smith GM. 2007. Analyzing ecological data. Springer.
Zuur AF, Ieno EN, and Elphick CS. 2010. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution, 1(1): 3–14.
Supplementary material
Supplementary Material 1 (DOCX / 217 KB)
- Download
- 216.32 KB
Information & Authors
Information
Published In

FACETS
Volume 6 • Number 1 • January 2021
Pages: 1266 - 1284
Editor: Mark Mallory
History
Received: 23 September 2020
Accepted: 3 April 2021
Version of record online: 29 July 2021
Copyright
© 2021 Fisher et al. This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Data Availability Statement
All relevant data are within the paper and in the Supplementary Material.
Key Words
Sections
Subjects
Authors
Author Contributions
JTF, FG, and S-LC conceived and designed the study.
FG, NA, JS, NA, and LN performed the experiments/collected the data.
JTF, AU, JAM, HWF, and SF analyzed and interpreted the data.
JTF and S-LC contributed resources.
All drafted or revised the manuscript.
Competing Interests
The authors have declared that no competing interests exist.
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Jason T Fisher, Fabian Grey, Nelson Anderson, Josiah Sawan, Nicholas Anderson, Shauna-Lee Chai, Luke Nolan, Andrew Underwood, Julia Amerongen Maddison, Hugh W. Fuller, and Sandra Frey. 2021. Indigenous-led camera-trap research on traditional territories informs conservation decisions for resource extraction. FACETS.
6: 1266-1284.
https://doi.org/10.1139/facets-2020-0087
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
Cited by
1. Indigenous‐led research on traditional territories highlights the impacts of forestry harvest practices on culturally important plants
2. Characterizing Sparse Spectral Diversity Within a Homogenous Background: Hydrocarbon Production Infrastructure in Arctic Tundra near Prudhoe Bay, Alaska
3. Disturbance‐mediated changes to boreal mammal spatial networks in industrializing landscapes
4. Grey wolves (Canis lupus) shift selection of anthropogenic landscape features following predator control in the Nearctic boreal forest
5. Native prey, not landscape change or novel prey, drive cougar (
Puma concolor
) distribution at a boreal forest range edge
6. How landscape traits affect boreal mammal responses to anthropogenic disturbance
7. Camera trapping in ecology: A new section for wildlife research
8. Integration of aerial surveys and resource selection analysis indicates human land use supports boreal deer expansion
9. Shifts in diel activity of Rocky Mountain mammal communities in response to anthropogenic disturbance and sympatric invasive white-tailed deer
10. A synthetic review of terrestrial biological research from the Alberta oil sands region: 10 years of published literature
