Introduction
Sea urchin populations are rapidly expanding across shallow rocky reef habitats in many temperate regions (
Filbee-Dexter and Scheibling 2014). Urchins can reach high densities and form feeding (grazing) fronts that rapidly overgraze macroalgae, including bioengineering kelps (
Filbee-Dexter and Scheibling 2014;
Frey and Gagnon 2015;
Ling et al. 2015). The proliferation of sea urchins, and the associated expansion of barrens, have been primarily linked to the over-exploitation of natural predators such as large groundfish and sea otters (
Jessup et al. 2004;
Pederson and Johnson 2006;
Bonaviri et al. 2009;
Sangil et al. 2012), but also to climate perturbations (e.g., heat waves) and disease-driven food web changes (
McPherson et al. 2021;
Smith et al. 2021). As kelp beds transition to barrens, habitat complexity, sediment accumulation and the availability of 3D-structures and shade decrease markedly, whereas wave exposure increases (
Reed and Foster 1984;
Rosman et al. 2007;
Watanabe et al. 2016;
Layton et al. 2019;
Morris et al. 2020), and this limits both food sources and refugia. This shift to a more simplified ecosystem can impose strong “environmental filters”, defined as a set of environmental conditions that select a subset of species from a regional species’ pool (
Lebrija-Trejos et al. 2010). Thus, the presence and absence of kelp may differentially select for subsets of traits or phenotypes (
Kraft et al. 2015) and, hence, lead to shifts in assemblage structure as well as the functional and genetic structure of populations.
The paucity of food (including kelp) in sea urchin barrens suggests that there is a fundamental difference in the selection pressures on sea urchin phenotypes as compared to those in kelp habitats (
Benjamin et al. 2010;
Hollins et al. 2018;
Duncan et al. 2019). Multiple species of macroalgae are commonly present in kelp habitats, and support preferential and selective feeding by grazers, whilst grazers in barrens often experience prolonged starvation and rely on drift kelp, filamentous algae, and biofilms for their nutrition (
Vanderklift and Wernberg 2008;
Filbee-Dexter and Scheibling 2014;
Renaud et al. 2015). The quantity and quality of food influences metabolic rate, which ultimately controls the pace of life and underpins an organism’s physiology and functioning (
Brown et al. 2004;
Huey and Kingsolver 2019;
Norin and Metcalfe 2019). Metabolic rate is the sum of all life-sustaining chemical reactions that create and use energy, and is typically estimated by measuring oxygen consumption (
). Metabolic plasticity is often observed under different environmental conditions and in relation to food (
Norin and Metcalfe 2019). Therefore, metabolic rates may differ between sea urchins living in kelp and barren habitats because of contrasting food availabilities and quality.
Understanding how metabolic rates are shaped by a species’ habitat also has implications in the context of climate change and population-level resilience. Organisms that cannot meet their basic energetic needs because of resource limitations may not be able to regulate and optimize their metabolic response to environmental stress. Metabolic rate can be used as a proxy to estimate an organism’s overall physiological state and to characterize its sensitivity to environmental change (
Silbiger et al. 2019), since metabolic rate fuels all organism functioning and is strongly temperature dependent (
Boltzmann 1872;
Gillooly et al. 2001;
Dell et al. 2011). Populations with different physiological trait distributions can respond differently when exposed to challenging thermal conditions, such as heat waves (
Padfield et al. 2016;
Silbiger et al. 2019). For example, two populations with different thermal tolerance ranges, temperature optima (where performance is maximal), and thermal safety margins (i.e., the difference between a species’ optimal temperature and its critical upper thermal limit) may respond differently to the same heat wave event, with only one population experiencing adverse effects. Yet, there has been limited research into how habitat shifts may shape physiological trait distribution within coastal populations (but see:
Bernhardt and Leslie 2013;
Miller and Dowd 2019;
Spindel et al. 2021) and what the implications are for climate resilience.
The northwest Atlantic represents a model system where green sea urchin (S
trongylocentrotus droebachiensis (O.F. Müller, 1776)) populations typically reach high densities, and can transform kelp habitats into extensive barrens (
Scheibling and Hatcher 2001). Green sea urchins in this region play a key ecological role as consumers, and exert strong top-down control on marine communities by removing foundational kelps (
Scheibling et al. 1999;
Gagnon et al. 2004;
Scheibling and Lauzon-Guay 2007;
Frey and Gagnon 2015). Even so, kelp beds and kelp patches of mainly
Alaria esculenta and
Laminaria spp. are present in the northwest Atlantic, in areas where sea urchin populations die off cyclically because of disease outbreaks or where high currents limit sea urchin grazing (
Keats et al. 1990;
Feehan and Scheibling 2014;
Frey and Gagnon 2016). This system provides an opportunity to directly compare sea urchin populations in terms of their physiology from these adjacent, yet contrasting, habitats.
In the present study, we use a standardized experimental approach to, first, determine whether green sea urchins from barren and kelp habitats differ in their realized physiological state (metabolic rate and thermal sensitivity) by measuring their absolute, mass-independent, and mass-specific
over a range of temperatures. Absolute
estimates the energy required per unit time to maintain biological functions, whilst mass-specific
gives metabolic rate scaled to the organisms’ mass (
Peters 1983;
Brown et al. 2004). Because absolute
and body mass are typically correlated (
Brown et al. 2004), and because sea urchin mass itself may vary across barrens and kelp habitats, we also calculate mass-independent
to compare populations without the confounding effect of body mass. Second, we test whether the thermal sensitivity of metabolism differs between sea urchins from barren and kelp habitats. Third, we compare two experimental approaches to investigate the effects of short-term increases in sea water temperature on urchin metabolism: Assay Method 1 an “acute” temperature exposure protocol, where sea urchins are transferred to a novel (stable) temperature, and oxygen consumption is measured at that temperature; and Assay Method 2 a temperature “ramping” (dynamic) exposure protocol, in which the same set of individuals are exposed to stepwise increases in temperature (
Terblanche et al. 2007). The latter experiment allowed us to construct thermal response curves (TRCs) for each individual (
Huey and Stevenson 1979) and enabled us to evaluate an individual’s response to a temperature gradient. We used these two approaches to test if the different methods result in similar, or different, temperature-dependent changes in
and to examine whether “heat-hardening” (i.e., an increase in heat tolerance following a sub-lethal exposure to elevated temperatures (
Maness and Hutchison 1980)) occurs during temperature ramping.
Results
Sea urchin density averaged 25 ± 9 individuals per 0.25 m
2 in barrens and 7 ± 5 individuals per 0.25 m
2 in areas with kelp across the three sites (26 ± 7 vs. 6 ± 7 at Biscayan Cove, 22 ± 13 vs. 8 ± 4 at Tors Cove and 26 ± 9 vs. 7 ± 5 at Bauline, in kelp vs. barrens, respectively). Across the three sites, green sea urchins from kelp habitats had a greater overall mass (by 8%; two-way ANOVA:
F(1,2) = 17.80,
p < 0.01), more metabolically active tissue (i.e., higher AFDM values by 48% (two-way ANOVA:
F(1,2) = 138.24,
p < 0.01)) and more inorganic mass (by 16%; two-way ANOVA:
F(1,2) = 23.66,
p < 0.01) than sea urchins from barrens (
Supplementary Fig. S4,
Tables S5 and
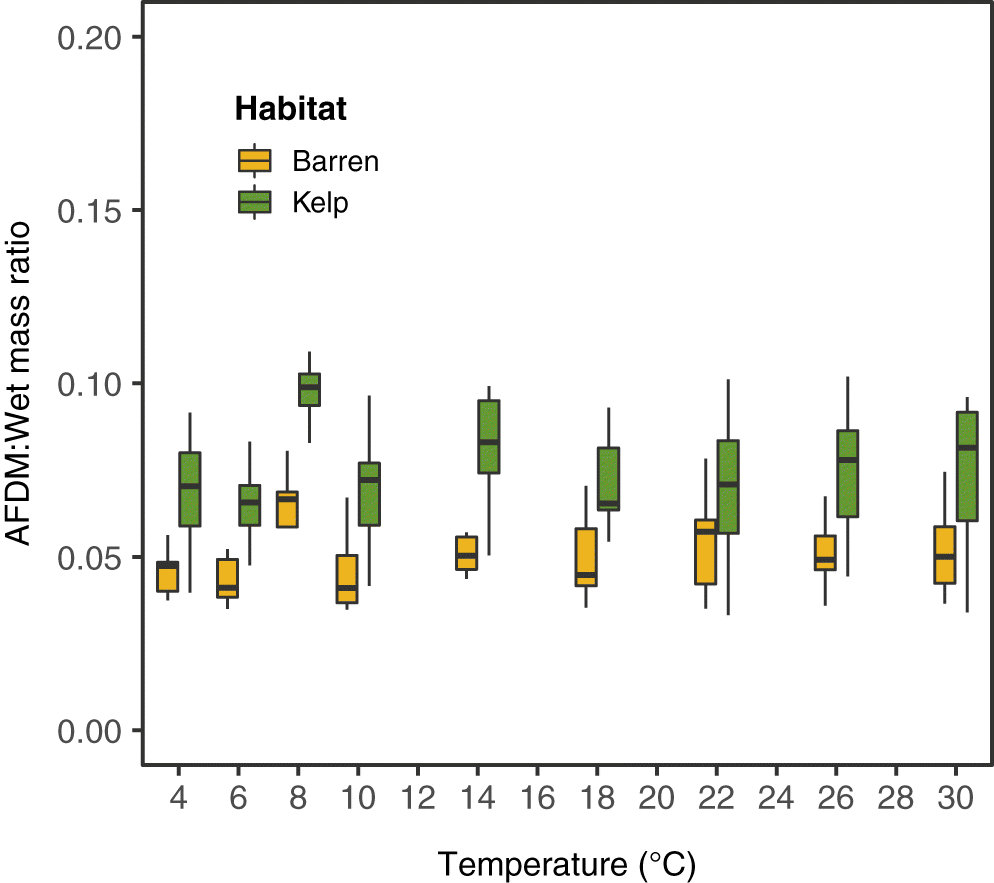
S6). Green sea urchin masses ranged across the sites from 23.3 g to 113.4 g (wet mass) and 0.9 g to 8.5 g (AFDM) in kelp and 15.2 g to 99.0 g (wet mass and) and 0.8 g to 5.3 g (AFDM) in barrens. Sea urchins from kelp habitats also had significantly higher AFDM to wet mass ratios (two-way ANOVA:
F(1,2) = 47.15,
p < 0.01;
Fig. 2;
Supplementary Table S5,
Fig. S5) than individuals from barrens.
Green sea urchin populations from barren and kelp habitats differed in their oxygen consumption (
Figs. 3 and
4). Overall, sea urchins from kelp habitats had significantly higher absolute
values (by 8%–78%) than sea urchins from adjacent barrens (
Figs. 3 and
4;
Supplementary Table S1;
Fig. S7) across the three study sites (
Fig. 1), and this pattern was generally consistent across temperatures (
Figs. 3 and
4). This difference was in part due to body mass (
Table S1), as urchins from kelp habitats had a higher wet mass and a greater AFDM:wet-mass ratio (
Fig. 2;
Supplementary Figs. S4 and
S5). However,
per g of AFDM was, in fact, lower as compared to sea urchins from barren habitats (
Supplementary Figs. S9 and
S10). Further, after accounting for body mass, the mass-independent
of sea urchins from kelp habitats was still significantly higher than that of sea urchins from barrens (
Figs. 3A and
3B;
Supplementary Table S1,
Figs. S7C and
S7D).
The temperature sensitivity (i.e., Q
10 value) of sea urchin
was significantly higher in animals from barrens than those from kelp habitats over the cold temperature range (Q
10 4–14 °C; one-way ANOVA:
F(1,6) = 27.47,
p = 0.002,
Supplementary Table S3), but only when assessed using the temperature ramping protocol (
Table 2,
Figs. 5A and
5C). Similarly, the temperature sensitivity of
in sea urchins from barrens was significantly higher in the warm temperature range (Q
10 14–26 °C) when measured using the acute protocol (one-way ANOVA:
F(1,4) = 11.35,
p = 0.028;
Supplementary Table S3). Mean Q
10 values ranged from 1.50 to 3.14, and from 1.31 to 1.84 across the two temperature ranges (4–14 °C and 14–26 °C, respectively) when sea urchins were exposed to the acute protocol. These values were similar to those measured using the ramping protocol, where Q
10 values ranged from 1.72 to 2.69 and from 1.46 to 1.93, respectively.
generally peaked between 26 and 30 °C when considering all site by habitat combinations (
Table 2), with urchins inhabiting areas with kelp having significantly (by 26%) higher maximum
values (one-way ANOVA;
F(1,7) = 8.008,
p = 0.025;
Fig. 5E;
Supplementary Table S3) than sea urchins from barrens. Despite the finding that sea urchins from kelp habitats had higher values for MMR
T, there was no significant difference in the temperature-induced metabolic scope (AS
T,
p = 0.254;
Fig. 5F,
Table 2;
Supplementary Table S3). There were also no significant differences in values of MMR
T or AS
T when standardized for wet mass (MMR
T,
p = 0.946; AS
T,
p = 0.397) or AFDM (MMR
T,
p = 0.090; AS
T,
p = 0.123), although sea urchins from kelp habitats had lower MMR
T and AS
T values than sea urchins from barren habitats when standardized for AFDM (
Supplementary Fig. S11). Overall, the
of Biscayan Cove urchins did not differ significantly when measured using the two methods (acute temperature protocol vs. the ramping protocol:
Fig. 6,
Table S2,
Fig. S8).
Discussion
We showed that green sea urchins from barren and kelp habitats in Newfoundland differ in their realized physiological states, with those from kelp habitats having higher metabolic rates, but reduced temperature sensitivity, compared to urchins from barren areas. The average urchin from areas with kelp consumed 8%–78% more oxygen than urchins from areas without kelp (i.e., barrens), across the range of typical ocean temperatures. The higher overall energetic requirements of sea urchins in kelp habitats were in part due to their greater mass. Yet, significant differences remained between sea urchins from areas with and without kelp when their mass-independent oxygen consumption rates were compared, which indicates that metabolic plasticity exists between habitat types regardless of mass effects. In contrast, when considering metabolism standardized per gram of animal, (i.e., AFDM) sea urchins from kelp habitats consumed less oxygen than barren urchins, which suggests a larger investment in energy-storing tissues with low oxygen demand in populations from areas with kelp. We conclude that sea urchin populations from kelp and barren habitats have fundamentally different mass-specific and mass-independent energy requirements and, hence, represent ecologically distinctive “units”. Such distinct populations may respond to, and interact with, their environment in unique but predictable ways.
Variation in individual energy requirements is ecologically important because biomass–energy relationships and the energetic status of a population form the basis of an organisms’ ability to respond to environmental variability. Energetic models, such as the metabolic theory of ecology (
Brown et al. 2004) or dynamic energy budget models (
Kooijman 1986), are built upon mass-energy scaling laws and are commonly used to predict organismal responses to environmental variability. Yet, most energetic models are average-based and predict a species’ mean response (rather than population specific responses) with unexplained, but often substantial, variation around the mean (
Saito et al. 2021). This can lead to inaccurate predictions. Integrating habitat-based energy relationships into energetic models could explain some of this variation, and improve the forecasting of a species’ vulnerability to environmental change.
The differences we detected in the metabolic performance of green sea urchins from barrens and kelp habitats presumably relate to habitat characteristics and the physical challenges and environmental filters that each habitat presents (as summarized in the Introduction). Yet, differences in food availability or quality emerge amongst a number of abiotic and biotic differences as the most likely driver of differences in the energetic demand and physiology of sea urchins (
Mueller and Diamond 2001;
Huey and Kingsolver 2019). This is because populations in kelp habitats have access to nutritious and rich food sources, whilst high-density urchin barren populations experience prolonged periods of starvation and compete for scarce food sources such as drift kelp, encrusting algae, and biofilms (
Norderhaug et al. 2003;
Vanderklift and Wernberg 2008;
Filbee-Dexter and Scheibling 2014;
Renaud et al. 2015;
Wells et al. 2017). Increased competition for scarce food resources in barrens intensifies food shortages, and this also appeared to be the case in the present study. Sea urchin densities were over three-fold higher in barren areas than in kelp areas across all sites.
Organisms that cannot meet their basic energetic demands because of reduced food availability or quality, or starvation, have limited capacity to regulate and optimize their metabolic response to environmental change (
Boersma et al. 2008;
O’Connor et al. 2009). Compared to sea urchins from kelp habitats, urchins from barrens in our experiments had lower absolute MMR
T values (by 26%), and their
was also more sensitive to increasing temperature (i.e., they had higher Q
10 values). Perhaps green sea urchins from barrens are more sensitive to temperature change because prolonged starvation reduces their ability to maintain homeostasis as a lower underlying plasticity of cellular traits cannot buffer for environmental change, and this leads to stronger temperature-induced metabolic responses (
Brett et al. 1969;
Huey and Kingsolver 2019). Although data on the longevity of the barrens’ state at these specific sites is not available, personal observations indicate that these barrens have existed at least since 2019, and urchin barrens are a persistent community state across the majority of the northwest North Atlantic (
Adey and Hayek 2011;
Frey and Gagnon 2015), suggesting extended starvation is likely. Additional studies on differences in protein expression in response to heat stress (e.g., heat shock proteins) between barren and kelp populations could prove insightful. It has been previously reported that various ectotherms lower their preferred body temperatures (reviewed in
Angilletta 2009) and metabolic rates (
Schuster et al. 2019) under starvation or reduced food regimes.
Green sea urchins in kelp habitats had larger body masses, more metabolically active tissue, and more inorganic (ashed) mass in comparison to urchins of the same test size from barrens (which contain relatively more water for a given body mass) across our study sites. Limited food availability in sea urchin barrens may limit urchins from shunting energy into tissue growth and storage. Sea urchins in areas with kelp, by contrast, may invest more energy into the growth of metabolically active tissues, but also the development of robust and larger body structures (e.g., ossicles or jaw length) and gonad growth (
Meidel and Scheibling 1998;
DeVries et al. 2019). This hypothesis is supported by our findings of relatively lower
, MMR
T and AS
T values in urchins from kelp compared to barren urchins when standardized to the mass of metabolically active tissue (i.e., AFDM). Greater investment in energy stores (e.g., lipids and glycogen) that consume little oxygen would explain why urchins from kelp habitats consume less oxygen per unit mass. Evidently, the internal structures of sea urchins from barren and kelp habitats are different, even though the populations appear visually similar (at a macroscopic level).
Variation in the body structure of green sea urchins from barrens and kelp may also emerge because of differing abiotic conditions across the two habitats. For example, kelp forests can alter local pH and dissolved oxygen (
Cornwall et al. 2013;
Krause-Jensen et al. 2016) and influence hydrodynamics, which in turn changes the residence time of chemically altered seawater (
Gaylord et al. 2012;
Hirsh et al. 2020). Thus, kelp forests present unique biochemical habitats compared to areas where kelp are absent. As such, areas with productive kelp have been suggested to act as deoxygenation and acidification refugia relative to surrounding waters (
Frieder et al. 2012). Thus, our finding that barren urchins have lower inorganic masses than kelp urchins suggests that a compelling direction for future investigations is whether differences in seawater biochemistry between barrens and kelp beds affect calcification processes in sea urchins (e.g.,
Hoshijima and Hofmann 2019).
We also report that the temperature at which oxygen consumption (
) of green sea urchins peaks (
Tmax; 26–30 °C) exceeds summer maximal coastal temperatures in Newfoundland, where sea temperatures typically reach 14 °C, and rarely exceed 20 °C (
Frey and Gagnon 2015;
Bélanger and Gagnon 2020). Thus, it is unlikely that ≥26 °C represents the realized maximum performance for this species, as longer-term experiments with green sea urchins show signs of deterioration at temperatures greater than 15 °C, and grazing rates rapidly decline above 12 °C (
Frey and Gagnon 2015). Instead, the temperatures where aerobic scope is highest would give a better indication of the optimum for sea urchin physiological performance and would likely lie well below the temperature where
peaks. In addition, the impact of disease dynamics on sea urchin performance under warming scenarios needs to be evaluated to predict how populations will fare in the future (but see:
Scheibling et al. 1999;
Lafferty et al. 2004;
Lester et al. 2007). Disease dynamics in high-density urchin barrens may interact with the heightened temperature sensitivity of barren urchins (i.e., impaired physiological states due to disease may further modify the shape of thermal response curves, or increased temperature sensitivity may increase disease vulnerability), leading to reduced population performance. Collectively, these data show that TRCs alone (and the T
max value) have limited capacity to predict population performance in the wild, or at what temperatures urchins begin to be impacted under slower rates of warming than in our experimental protocols. We advise caution with regards to applying temperature tolerance data from rapid, short-term exposures to species’ population models that predict species’ success in future climates and inform conservation decisions.
In this study, we also found that exposure to an acute temperature protocol versus a ramping temperature protocol yielded similar
values (for this urchin species and ecological context). This was unexpected as there is much debate about which physiological assay designs are most suited to particular lines of investigation, and different assay protocols can lead to different physiological responses (
Terblanche et al. 2007;
Bates and Morley 2020). In the acute protocol, individuals were moved from the ambient holding temperature to a target temperature, and each individual was used for one independent measurement. Consequently, there was a greater temperature “shock” at increasingly warmer temperatures. Acute approaches are also both time and replication intensive, as each individual organism is only exposed to a single temperature challenge. By contrast, “ramping” approaches repeatedly measure the same individuals across multiple temperature steps. In such approaches, cumulative temperature effects as organisms are exposed to longer durations of heat stress can limit the inferences from the results of ramping approaches (
Overgaard et al. 2012).
Accumulated temperature effects may also lead to “heat-hardening”, where thermal tolerance is impacted by previous sub-lethal heat exposures during ramping, which can impact rate measurements (
Dahlgaard et al. 1998;
Kelty and Lee 2001). However, many researchers now recognize that ramping protocols that use ecologically relevant rates of heating (e.g., that reflect acute temperature changes in smaller water bodies or tide pools or seasonal changes in coastal water temperatures) are the most appropriate method when the question relates to species in their natural environment, and the goal of the study is not specifically to study the maximum or minimum temperature at which a particular physiological mechanism fails (e.g.,
Leeuwis et al. 2019;
Zanuzzo et al. 2019;
Gamperl et al. 2020). Further, in this study there was no evidence of heat-hardening (when urchins were exposed to increasing temperature steps), or a time or cumulative heat load effect on
. The slightly higher
values with the ramping protocol may have been related to differences in ambient seawater temperature at the time of sea urchin collection (urchins collected for the acute protocol in mid-August (∼13 °C ambient seawater) versus collection for the ramping protocol during early November (∼7 °C ambient seawater)). This interpretation is supported by rate differences at the first temperature step (4 °C), where stress resulting from incremental temperature ramping had not accumulated yet (i.e., the first temperature step of a ramping protocol is equivalent to that of an acute protocol). Impacts of heat-hardening would manifest in diverging
values at higher temperatures during the ramping protocol, relative to
values recorded during the acute protocol, but we found no evidence of this in the present study.
Our results suggest that, at least for green sea urchins, the acute and ramping protocols provide comparable data and do not lead to different oxygen consumption values when the same equipment is used. Even so, researchers should match their experimental design to their research question, in particular when laboratory assays are used to infer or predict climate vulnerability and when heat-hardening, acclimation, and adaptation are fundamentally important and ecologically relevant (
Bates and Morley 2020). Therefore, in some cases faster ramping approaches, which require less time and fewer replicates, may be a practical choice for comparison when the goal is to compare amongst many individuals and species. Developing realistic temperature ramping protocols that reflect current rates of change experienced by wild populations, or those predicted in the future (e.g.,
Zanuzzo et al. 2019;
Gamperl et al. 2020), are crucial to produce accurate bounds on which to base predictions.
Overall, habitat type emerged as a driver of population-level variations in realized physiology. Green sea urchins (and likely other sea urchin species) from barrens are ecologically different units than those from kelp habitats in terms of their metabolic responses to temperature change. Our findings have important implications for the application of energy-based models (e.g., metabolic theory of ecology or dynamic energy budget models) that aim to understand and predict a species’ vulnerability under climate change. We show that habitat may play a fundamental role when considering organisms as energetic units and in explaining differences between individuals. Testing our observations in different species that occupy several distinct habitats (including species occurring in both forests and deforested areas on land) could reveal whether habitat complexity produces consistent energetic sub-units within species that can be integrated into forecasting approaches.