Introduction
An important goal in ecology is the development of general theories that can explain observed spatiotemporal variation in biological communities (
Pearson and Rosenberg 1978;
Hubbell 2005;
Huston 2014). These then enhance our ability to predict outcomes of natural or anthropogenic disturbances (
Pearson and Rosenberg 1978;
Orwin et al. 2016), as well as of restoration following disturbance (
Campbell et al. 2019a). Frameworks for theory on forces underlying community composition and dynamics include niche vs. neutral dynamics (
Hubbell 2005) and the designation of such forces into categories related to habitat availability, species availability, and species performance once in the habitat (such as predation, competition, and tolerance to environmental conditions) (
Pickett et al. 1987;
Meiners et al. 2015), among other frameworks.
Here, we examine possible species performance forces (predation, competition, and response to certain abiotic conditions) underlying community structure. For instance, environmental or abiotic variables, such as water salinity, temperature, or sediment properties, coupled with variations in exhibited tolerances of organisms for these conditions (
Stillman 2002;
Lu et al. 2008;
Dashtgard et al. 2014;
Sizmur et al. 2019) can play an important role in structuring communities (
Kelaher et al. 2001;
Ferguson et al. 2013;
Gerwing et al. 2022a). As well, interactions among the living components of an ecosystem can affect communities, namely top-down predation, which in our analysis, and as discussed below, may be indistinguishable from bioturbation (
Heck Jr. and Valentine 2007;
Hughes et al. 2014;
Johnson et al. 2014) and bottom-up forces, such as the availability and competition for nutrients (
Davis et al. 2014;
Schuldt et al. 2014;
Pilditch et al. 2015a). Many communities appear shaped by a combination of top-down and bottom-up forces (
Hamilton et al. 2006;
Bracken et al. 2014;
Greenville et al. 2014). Added complexity is observed in systems with mesopredators (mid-trophic level predators) (
Prugh et al. 2009), which act both as predator and prey (
Ambrose Jr. 1991;
Marczak et al. 2011). These species can exert strong structuring influences upon a community via predation, bioturbation, and competition in a middle-out manner (
Elmhagen and Rushton 2007;
Quijón and Snelgrove 2008;
Cunningham et al. 2020). Designing studies to elucidate the individual role of abovementioned forces in structuring a community is relatively straightforward; however, it is more difficult to quantify the relative importance of these forces, occurring concurrently, in determining community structure and dynamics (
Wootton 1994;
Agrawal et al. 2007;
Dray et al. 2012;
Gerwing et al. 2022b).
While trophic and habitat-related abiotic variables are known to influence community structure in many ecosystems, some systems appear to be only minimally influenced by them. For instance, ephemeral freshwater habitats, such as floodplains, can act as resource-pulse ecosystems (
Crook et al. 2020;
Nelson et al. 2021). In such systems, amply available resources, whose availability is temporally limited, trigger a cascading response throughout the ecosystem, minimizing the controlling influence of competition for resources, predation, and an individual species’ preference for abiotic conditions (
Tilman 2004;
Letnic and Dickman 2010;
Greenville et al. 2014). In resource-pulse systems, more research is required to better understand how these communities are structured. Another study, pertinent to our current study, focused on intertidal mudflats in the upper Bay of Fundy (NB and NS, Canada).
Gerwing et al. (2016) assessed the relative importance of top-down, bottom-up, middle-out, and sediment-related abiotic variables in structuring the infaunal community (invertebrates living in the sediment). They observed that while top-down, bottom-up, middle-out, and abiotic variables accounted for a statistically significant proportion of the observed community variation (1%–11%), the majority of that variation (79%) was accounted for by spatial factors, such as study site or patch.
Gerwing et al. (2016) suggested two sets of hypotheses for their observed patterns. First, high abundance of resources on the expansive Bay of Fundy mudflats may both minimize bottom-up control (e.g., by limiting competition for nutrients from sediment organic matter and benthic diatoms) and support a sufficiently dense population of potential prey items to dilute the importance of top-down and middle-out predation/bioturbation. Furthermore, the relatively low importance of abiotic variables in structuring the infaunal community could be a result of generally low horizontal physical heterogeneity observed on their study sites, as well as high nutrient concentrations that limit the influence of abiotic factors by attracting animals to patches with abiotic characteristics that would normally reduce occupancy. Pulses of amply available resources may result in intertidal mudflats operating as a resource-pulse ecosystem. Secondly,
Gerwing et al. (2016) also hypothesized that since mudflats are a relatively benign environment for organisms adapted to living in mud, the inhabiting communities were not predominantly structured by top-down, bottom-up, middle-out, or abiotic factors. Instead, these communities may reflect priority effects or a “first come, first served” process (
Sutherland 1974;
Connell and Slatyer 1977). Initial delivery of larvae or colonization by juveniles and adults may play a predominant role in explaining the observed spatiotemporal variation in this type of community (
Snelgrove et al. 1999;
Pilditch et al. 2015a;
2015b). In contrast to the resource-pulse hypothesis, priority effects imply some competition for resources.
While these sets of hypotheses are intriguing, there are questions about their applicability to other tidal flat systems. The Bay of Fundy is home to the largest tides in the world, a phenomenon that results in considerable amounts of sediment being suspended in the water column each day (
Wu et al. 2011). Sediment dynamics may be partly responsible for the relatively limited biodiversity exhibited on the upper Bay of Fundy mudflats, by filtering out species unable to survive in such situations. For instance, the community of these Bay of Fundy mudflats is composed of roughly 10 infaunal taxa, 3 top-down predators, and 3 mesopredators (
Gerwing et al. 2015a). Conversely, mudflat communities on the Pacific coast of BC, Canada, are composed of roughly 39 infaunal taxa, 8 top-down consumers, and 12 mesopredators (
Campbell et al. 2020). Given the unique characteristics of Bay of Fundy mudflats, it is unclear whether the hypotheses of
Gerwing et al. (2016) can be applied to other mudflat or tidal flat communities. Moreover, field studies focused on infaunal community recovery from anthropogenic and natural disturbances along the Pacific and Atlantic coasts suggest that priority effects may not be the predominant force structuring these communities (
Norris et al. 2022). Instead, community trajectories may be strongly influenced by species availability, particularly regional species pools (
Beukema et al. 1999;
Thrush et al. 2003;
Gerwing et al. 2017a;
Campbell et al. 2019a;
Cox et al. 2019;
Gerwing et al. 2022c;
Norris et al. 2022). As such, more work is required to better understand the suite of forces structuring infaunal communities.
Our goal for the present paper was to use community and population level correlations to investigate the potential relative importance of top-down, bottom-up, middle-out, and abiotic variables in structuring the infaunal community of tidal flats (both sand and mudflats) of the north coast of BC (Supplementary Table S1). We hypothesized that, as observed in
Gerwing et al. (2016), top-down, bottom-up, middle-out, and abiotic variables would not associate well with the infaunal community. If top-down, bottom-up, middle-out, and abiotic variables have only a minor influence in structuring tidal flat communities on the Pacific coast, as inferred by
Gerwing et al. (2016) on the Atlantic coast, it is possible that similar processes are operating in other infaunal communities elsewhere, thus expanding our empirical understanding of the relative importance of the forces that structure soft-sediment intertidal communities.
Results
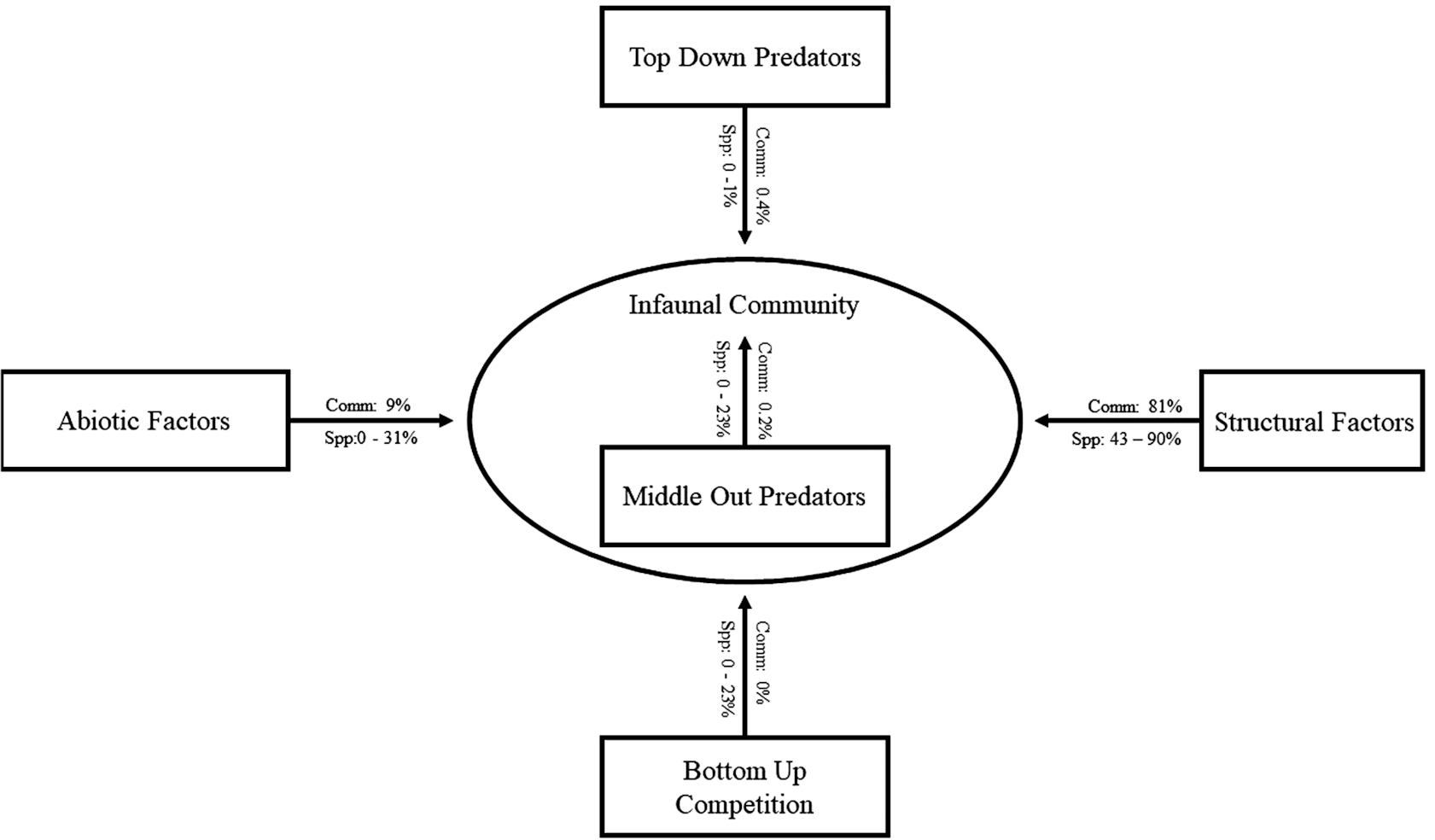
Structural factors accounted for most of the observed variation in the infaunal community (81%), with site (our largest spatial scale, 35%) and plot (our smallest spatial scale, 33%) accounting for the most (
Table 1). Transect, which was intermediate in spatial scale and integrated across intertidal depth, accounted only for ∼3% of the community variation. While most of the relationships central to our study were statistically significant, top-down consumers only accounted for 0.4% of the variation, middle-out predators 0.2%, and abiotic variables 9% (
Table 1). The covariate that accounted for the highest proportion of the variation was water salinity (7%). Bottom-up variables (nutrients) did not have a significant influence.
Individual taxa exhibited a similar trend, with the majority of the variation accounted for by structural factors (
Table 2). However, several key exceptions were noted, where covariates accounted for ≥10% of the observed variation in an infaunal taxon. These exceptions were correlations between water salinity and
Cumella vulgaris (16%),
Leptochelia spp. (28%), Oligochaeta (11%), Nematoda (12%), and
Nutricola tantilla (15%); sediment organic matter content correlated with
Nippoleucon hinumensis (22%) and
Capitella species complex (12%); algal cover correlated with
S. zonata (31%) and
Exogone lourei (17%); and sediment particle size correlated with
Macoma balthica (17%). Finally, a mixture of positive and negative correlations was observed between covariates and individual populations (
Table 2).