Introduction
Extreme changes in ambient temperature can push species outside of their thermoneutral zone and into constant thermal stress (
Khaliq et al. 2014;
Murali et al. 2023). The thermoneutral zone represents the range of ambient temperatures between the lower critical temperatures (LCTs) and upper critical temperatures (UCTs) at which an endotherm maintains a stable metabolic rate without regulatory changes in heat production or evaporative heat loss (
IUPS 1987). Below the lower critical temperature, a resting thermoregulating animal must increase its rate of metabolic heat production via shivering or nonshivering thermogenesis to maintain thermal balance (
IUPS 1987). However, above the UCT the rate of evaporative heat loss or metabolic rate of a resting thermoregulating animal must be increased by panting or sweating to maintain thermal balance (
IUPS 1987). Moreover, ambient temperatures will rarely be ideal for both adults and juveniles because juveniles have much higher and narrower thermoneutral zones than adults (
Whittow et al. 1987;
Mitchell et al. 2018). Subsequently, juveniles are more susceptible to cold and heat stress resulting in significant thermoregulatory costs and consequences for growth (
Weathers et al. 2000;
Mitchell et al. 2018).
The consequences of climate change and severe weather are a serious threat to seabirds because they impact over 170 million individuals worldwide (
Dias et al. 2019). Indeed, seabirds face demanding thermal conditions throughout the breeding season and during extreme events that can exceed their UCTs and fall below their LCTs (
Moe et al. 2009;
Choy et al. 2021). Seabird chicks are particularly vulnerable to extreme weather events because after achieving homeothermy, they typically remain in their nests until fledging. Consequently, chicks are exposed to all adverse weather that occurs during the breeding season regardless of whether they occupy open or burrow nests. For example, thousands of ground-nesting American White Pelican (
Pelecanus erythrorhynchos) chicks perished as a result of several extreme events that produced adverse extended cold, wet, and windy weather in North Dakota over a five-year period (
Sovada et al. 2014). Colonies of burrowing seabirds are also vulnerable to flooding, cooling, and collapse during extreme weather (
Glencross et al. 2021). Yet, similar to other reptile, mammal, and bird burrows, seabird burrow nests can moderate temperatures and provide a stable thermal environment (
Manuwal 1974;
Kulaszewicz and Jakubas 2018). Despite this thermal protection, chicks have elevated LCTs compared to adults; therefore, burrow temperatures often fall below chick LCTs and incur significant energetic costs (
Whittow et al. 1987;
Weathers et al. 2000;
Moe et al. 2009). Consequently, it is imperative to understand how environmental extremes change seabird nest microclimates, whether burrows provide thermal refuges for chicks and adults, and to monitor the implications if burrow temperatures further deviate outside seabird critical temperatures.
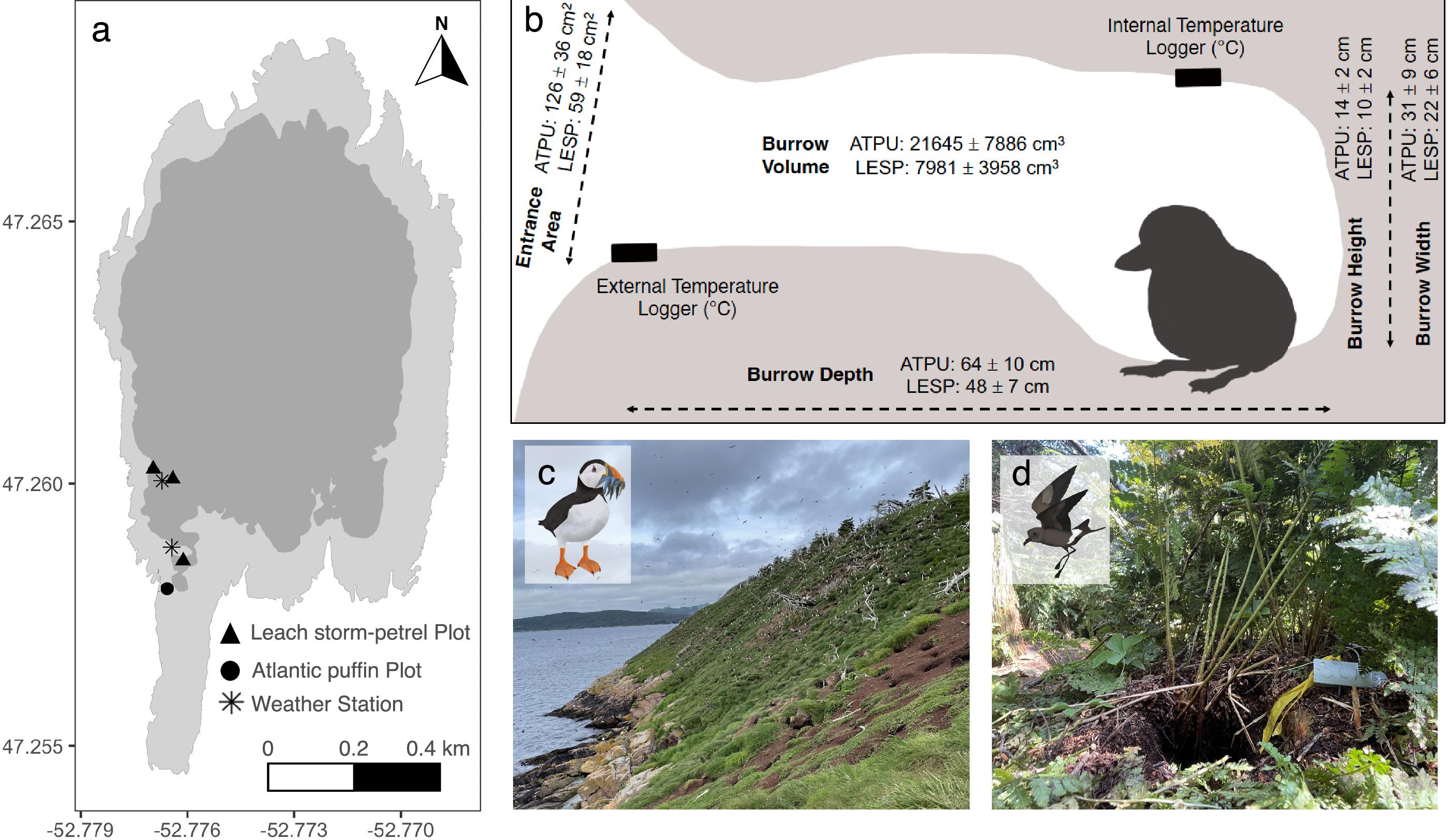
Newfoundland, Canada, hosts globally significant numbers of breeding seabirds (
Gaston et al. 2009). In the face of climate change, the area is predicted to experience more storms and extreme cold weather, especially if the Atlantic meridional overturning circulation collapses (
Trepanier 2020;
He et al. 2022;
Ditlevsen and Ditlevsen 2023). Two burrow-nesting species facing the growing threat of such extreme events are the Atlantic Puffin (
Fratercula arctica), a ca. 450 g Alcid which is classified as globally Vulnerable by the International Union for Conservation of Nature (IUCN) Red List (BirdLife
International 2018a;
Lowther et al. 2020), and the Leach’s Storm-petrel (
Hydrobates leucorhous), a ca. 45 g Procellariid which is assessed as Vulnerable by the IUCN and threatened by the Committee on the Status of Endangered Wildlife in Canada (
COSEWIC 2020;
BirdLife International 2018b;
Pollet et al. 2021). For example, during the breeding season, extreme cold weather caused a hypothermia-induced mass mortality of Atlantic Puffin chicks in Newfoundland (
Wilhelm et al. 2013), while Leach’s Storm-petrels, which breed later than Atlantic Puffins, are regularly exposed to hurricanes and cyclones towards the mid-late breeding season. It is speculated that these extreme events may flood or cool Leach’s Storm-petrel burrows, causing hypothermia or death (
Pollet et al. 2023). Yet, there is limited knowledge on the burrow microclimate of both species and whether nests provide a thermal refuge to adults and chicks during extreme cold weather events (
Lowther et al. 2020;
Pollet et al. 2021). Filling this basic biology knowledge gap can inform on the capacity of burrow systems to buffer temperature change during extreme events for these threatened species. Moreover, Atlantic Puffins and Leach’s Storm-petrels are excellent models to test how different burrow and habitat characteristics improve such resilience because they can nest in close proximity, yet occupy different habitats. In Newfoundland, the Atlantic Puffin predominantly selects nesting sites along steep grass-covered maritime slopes; therefore, burrows have no additional shelter and are exposed to high solar radiation, variable temperatures, and high wind speeds (
Lowther et al. 2002). By contrast, Leach’s Storm-petrels nest in a variety of island habitats ranging from open grass meadows to heavily-canopied forests, which may offer additional thermal protection from extreme events (
Huntington et al. 1996). Collectively, revealing such patterns could help inform habitat conservation measures that may be needed given increases in extreme events in the future.
Here, we (1) describe the inter- and intra-specific variations in Atlantic Puffin and Leach’s Storm-petrel burrow microclimates, and (2) test for the effect of external weather conditions on internal burrow temperatures. These objectives allow us to (3) quantify the proportion of time that burrows are cold thermal refuges for adults and chicks through the season and during extreme weather events. Finally, we (4) identify the features of burrows with superior buffering capacity during extreme cold events.
Discussion
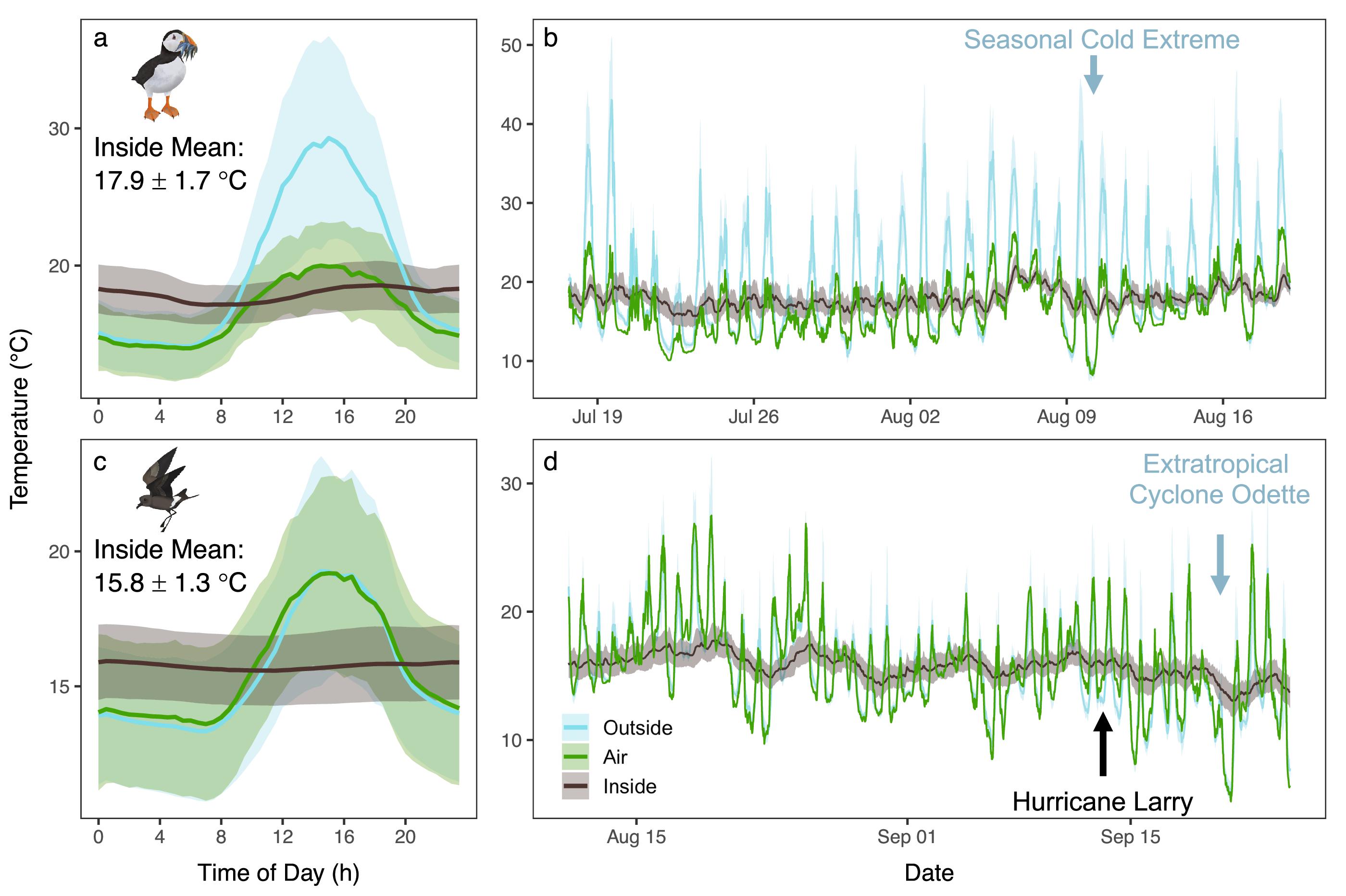
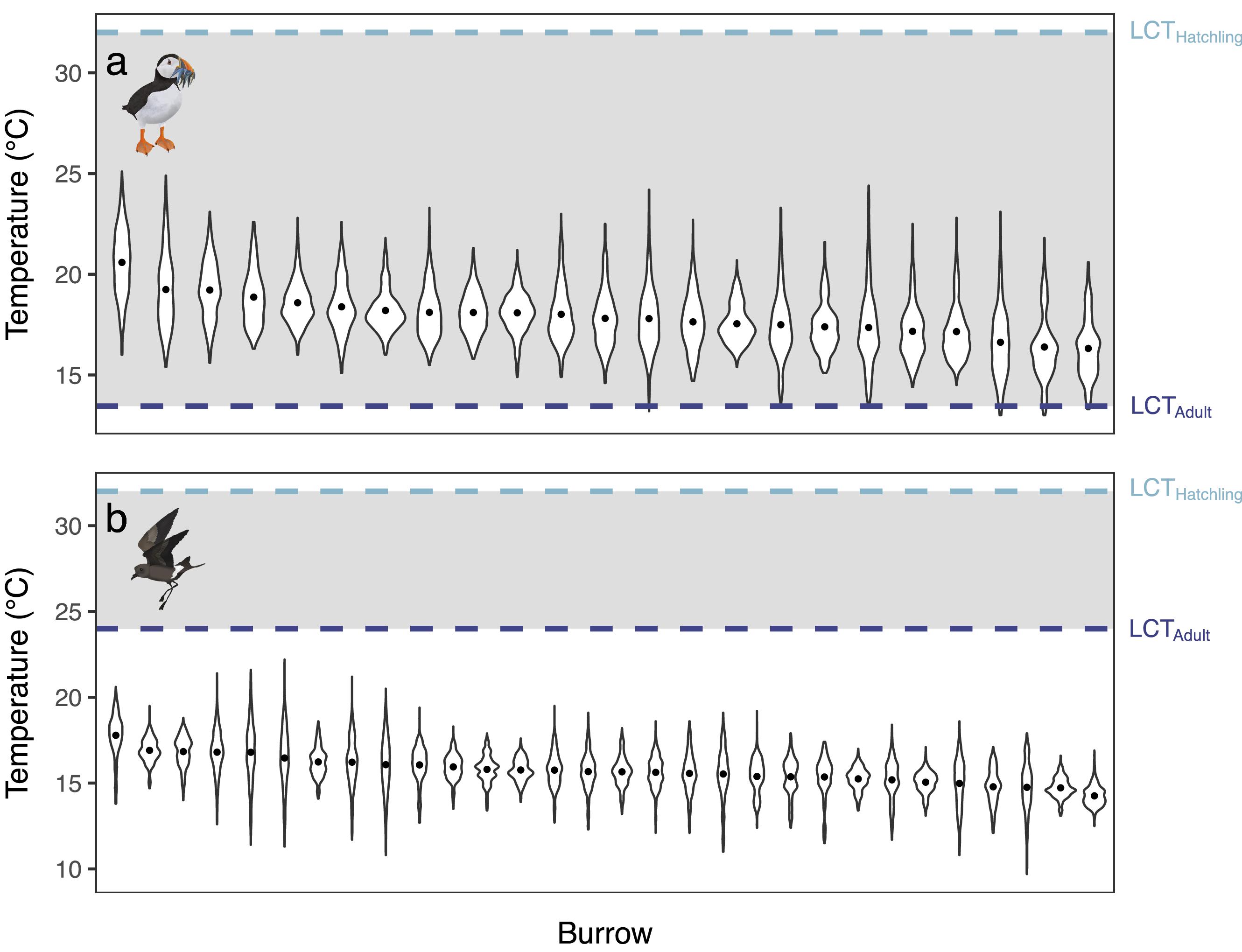
Here we revealed that both the Atlantic Puffin and Leach’s Storm-petrel actively breed in burrow microclimates that are below their LCTs. Specifically, burrow temperatures always fell below the LCTs of both adult and chick Leach’s Storm-petrels, and Atlantic Puffin chicks throughout the season. Yet, for adult Atlantic Puffins, the burrow temperatures were higher than their LCTs. Similar observations have been found in other burrowing auk and tubenose species in polar and tropical regions. For example, burrow temperatures of Dovekie (
Alle alle) breeding in the high arctic fell below adult lower critical temperature (
Gabrielsen et al. 1991). Similarly, in Antarctica, burrows of Snow Petrel (
Pagodroma nivea) were significantly colder than the lower critical temperature of both adults and chicks (
Weathers et al. 2000). Meanwhile, in the topics, burrow temperatures were within the thermoneutral zone of adult Wedge-tailed Shearwater (
Puffinus pacificus); however, burrow temperatures fell below chick LCTs (
Whittow et al. 1987).
Nesting in microclimates that are outside of the species’ thermoneutral zone may have energetic consequences for both of the seabird species. For example, based on our findings, Leach’s Storm-petrel adults and chicks, and Atlantic Puffin chicks may divert considerable amounts of energy toward thermoregulation. Indeed, endotherms incur significant thermoregulatory costs to maintain body temperatures when environmental conditions are outside of their thermoneutral zones (
Weathers et al. 2000). In adults, this translates to energy being diverted away from other metabolic processes, such as reproduction, while chicks divert considerable amounts of energy away from growth (
Kooijman 2010). Indeed, to maintain normothermy in burrows that are significantly lower than their lower critical temperature, Snow Petrel chicks must increase their resting metabolic rate 212% above thermoneutral levels (
Weathers et al. 2000). The diversion of energy towards thermoregulation and away from growth is a contributing factor that leads to burrow nesters often having slower growth and longer nest periods compared to other species, as observed in passerines and fulmarine petrels (
Martin and Li 1992;
Weathers et al. 2000;
Durant et al. 2005).
Little is known about the thermoneutral zone and the energetic cost of thermoregulation for adult and chick Atlantic Puffin and Leach’s Storm-petrels through the breeding season (
Ricklefs et al. 1980;
Bech et al. 1987;
Gabrielsen and Ellis 2001;
Ochoa-Acuña and Montevecchi 2002). Here, to understand whether burrow temperatures fall below the species’ LCTs, we used historical experimental measurements of critical temperatures from colonies between 44°N to 65°N which represent a static snapshot of a single day in the breeding season. We also extrapolated the lower critical temperature for adult Atlantic Puffins based on a regression between latitude and mass. Yet, higher latitude seabirds can persist at lower temperatures than species of similar mass from warmer climates, and a chick’s thermoneutral zone decreases with increasing mass through the breeding season (
Whittow et al. 1987;
Weathers et al. 2000;
Gabrielsen and Ellis 2001). Consequently, the static values for LCTs used in the present study may not fully capture the dynamic changes in adult and chick LCTs through the season within Witless Bay Ecological Reserve, located at 47°N. Future research could leverage non-invasive methods, such as a field metabolic rate approach or Dynamic Energy Budget Theory and NicheMapper modelling frameworks, to fill spatio-temporal knowledge gaps on changes in seabird thermoneutral zones through the breeding season and across their breeding ranges (
Kooijman 2010;
Dunn et al. 2018;
van der Meer et al. 2020;
Kearney et al. 2021). Such research could help understand the interplay between temperature, energetic costs of thermoregulation, growth and breeding success implications, and identify species with limitations to extreme weather events.
Our findings strongly support that access to suitable burrowing habitat could be important for burrow nesting seabirds during extreme weather events. We find that burrows provide a stable thermal environment and a refuge from cold because burrows acted as buffers that kept nests warmer than ambient temperatures during extreme cold events. Indeed, our results align with other studies on burrow-dwelling seabirds. For example, Cassin’s Auklet (
Ptychoramphus aleuticus) burrow temperatures remained stable on Farallon Island, California, despite large fluctuations in outside ambient temperature (
Manuwal 1974). Likewise, in the high arctic, Doviekie burrows were warmer than ambient air temperatures (
Kulaszewicz and Jakubas 2018). Burrows also provide other endotherms and ectotherms, such as pangolins, insects, and lizards, thermal protection from extreme temperatures by buffering lethal hot and cold exposure (
Bao et al. 2013;
Moore et al. 2018;
Sunday et al. 2014). Moreover, similar to the present study, occupying burrows provides a thermal refuge for a number of other species, and was further identified as an effective strategy to mitigate thermoregulatory costs. For example, while burrow temperatures often fall outside Pygmy Rabbit (
Brachylagus idahoensis) and Burrowing Owl (
Athene cunicularia) thermoneutral zones, their burrows provide a refuge from extreme temperatures because internal temperatures are closer to their thermoneutral zones than ambient temperatures (
Nadeau et al. 2015;
Milling et al. 2018b). Consequently, thermoregulation costs are lower within the burrow compared to above ground (
Milling et al. 2018b). Given that climate extremes are predicted to increase in frequency and intensity in the future (
Wingfield et al. 2017;
Harris et al. 2018), burrows may therefore provide a direct line of defence for seabird chicks against current and future extreme cold events, as predicted for other species (
Moore et al. 2018).
We found that the thermal response of burrows varied in different extreme weather events. Specifically, burrow buffering capacity was greatest during extreme cold weather events for both species. Yet, during the hurricane, there was minimal buffering within Leach’s Storm-petrels burrows because ambient temperatures were warmer than internal temperatures, thus indicating it will be important to monitor the impacts of variable extreme weather events in the future. Indeed, extreme event variability drives negative ecological responses across taxa and systems (
Maxwell et al. 2019). A factor that may be critical to research further in the context of burrowing species is extreme precipitation events. For example, burrows are vulnerable to flooding and collapse during extreme precipitation events which increases breeding failure energetic demands (
Wilhelm et al. 2013;
Tiller et al. 2000;
Glencross et al. 2021).
We further recorded large intraspecific variation in burrow temperatures, the extent that burrows were cold refuges, and burrow buffering capacity through the season and during extreme cold events. For example, there was a 5 °C difference in the average buffering capacity between burrows during extreme cold weather for both species. Similarly, there was around 4 °C difference in the mean burrow temperatures between burrows of both species through their breeding seasons. Indeed, a previous study has similarly reported large inter-nest temperature differences between Dovekie burrows in the high-Arctic (
Kulaszewicz and Jakubas 2018). Here, the interplay between air temperature and wind speed emerged as a key driver for the internal thermal microclimate of Atlantic Puffin and Leach’s Storm-petrel burrows, which is consistent with other studies. For example, Cassin’s auklet burrow temperatures fluctuated in proportion to the changes in ambient temperatures, and temperatures were buffered more within soil burrows compared to rock crevice nests (
Manuwal 1974). Similarly, external air temperature, wind speed, and wind direction determined the internal temperature of Wilson’s Storm-petrel (
Oceanites oceanicus) nests (
Michielsen et al. 2019). However, despite the large intraspecific variability in burrow buffering capacity, we found that burrow characteristics and canopy cover do not predict Atlantic Puffin and Leach’s Storm-petrel burrow buffering capacity during extreme cold weather. Given wind speed and ambient temperature were important correlates of internal burrow temperature for both species, a burrow’s insulation and protection from wind exposure may be key for predicting burrow buffering capacity during cold extremes. For example, smaller nest dimensions (entrance area), greater insulation, burrow orientation, and less wind exposure were important for establishing warmer thermal environments for Wilson’s Storm-petrels nesting in extreme cold in Antarctica (
Michielsen et al. 2019).
To further explore drivers of burrow microclimates and buffering capacity, future studies could improve nest dimension measurements through 3D scanning the complex internal shapes of seabird burrows, measuring the thermal microclimate throughout the burrow tunnel and nest with an array of temperature loggers, and incorporating additional model parameters, such as nest orientation and slope.
Given the complex responses of burrow microclimates to extreme events, quantifying how changes in a variety of external and internal environmental conditions will impact burrow-nesting seabirds is a key future direction. This will be particularly critical in Newfoundland since the frequency of extreme windy days have increased over the past decade (
Government of Canada 2022). Moreover, habitat management approaches may be needed to increase nest temperatures during extreme cold events. Artificial nest boxes show great promise for improving the breeding success of burrowing seabirds (
Libois et al. 2012;
Sutherland et al. 2014). Insulation or artificial heating of nests when temperatures fall below the species’ LCTs may be a way to protect chicks from future extreme cold events. Even so, special consideration of the nest box design will be imperative to prevent overheating or excessive cooling (
Lei et al. 2014;
Kelsey et al. 2016;
Fischer et al. 2018).