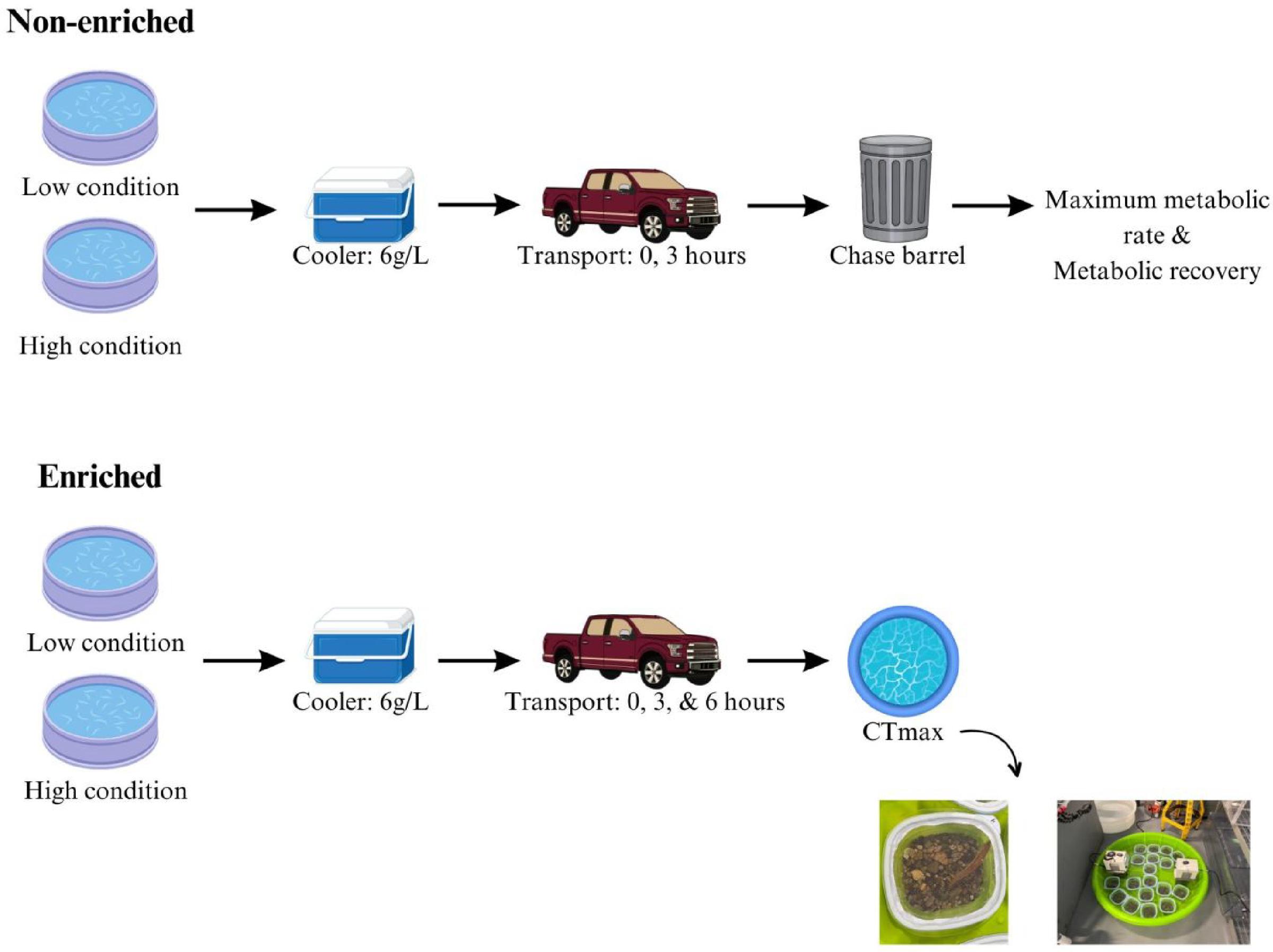
Introduction
Reintroduction is an important tool for the conservation and recovery of aquatic species at risk (
Seddon et al. 2007;
Cochran-Biederman et al. 2015;
Lamothe et al. 2019;
Drake et al. 2021). Reintroduction success ultimately hinges on the long-term survival and reproduction of released individuals. One of the most critical periods for reintroduced animals occurs during the first hours after release, when rapid acclimatization to a novel environment is required (
Näslund 2021). This period may be especially difficult because of the repeated stress imparted by reintroduction procedures (
Dickens et al. 2010). For fishes in particular, transportation can be particularly challenging, causing a suite of physiological changes due to handling, air exposure, confinement, motion during transport, changes in water quality parameters, and high conspecific densities (
Teixeira et al. 2007;
Sampaio and Freire 2016). Accordingly, the potential for components of the reintroduction process to induce behavioral and physiological stress must be carefully considered, and strategies must be implemented to minimize their negative consequences.
One promising strategy to alleviate some of the negative effects of transport is the provisioning of extra food in the weeks prior to transport to bolster condition, which may help increase physiological resilience. When fish encounter acute stressors (e.g., those associated with transport), the activation of the HPI (hypothalamic-pituitary-interrenal) axis triggers the production and circulation of glucocorticoids, energy-mobilizing hormones that shift energy resources away from regular physiological processes, redirecting them toward restoring homeostasis (
Barton et al. 2002). This stress-induced physiological response is energetically costly (Barton 2002); however, the extent of these negative costs may depend on the nutritional state of fish. For instance, food availability may improve physiological performance measures such as maximum metabolic rate (MMR) and thermal tolerance, two fitness-related measures of physiological resilience thought to be influenced by body condition (e.g.,
Pörtner and Peck 2010;
Norin and Clark 2016;
Turko et al. 2020). Accordingly, during the captive rearing stage of the reintroduction process, there is a unique opportunity to increase body condition through supplemental feeding or other dietary adjustments. Although much research has explored supplementation of specific dietary components such as carotenoids, probiotics, and glucan (see review in
Vanderzwalmen et al. 2019), there has been limited focus on overall condition factor. While energy storage and food availability can reduce energy demands during stressful events (
McEwen and Wingfield 2003), a better understanding of whether nutrition and dietary strategies can play a role in the success of reintroduction efforts is warranted.
Measuring ecologically relevant indicators that reflect the connection between the stress response and fish performance following transport is important to improve the success of reintroductions. MMR (and subsequent recovery) is an ecologically important indicator of physiological resilience, representing the upper limit at which oxygen can be delivered from the environment to the mitochondria within an organism’s tissues (
Norin and Clark 2016). At a minimum, organisms must allocate energy toward the maintenance of essential homeostatic mechanisms to sustain life—beyond this, any surplus can be allocated to other functions, albeit within the confines defined by MMR (
Fry 1971). In essence, MMR sets an upper boundary on an organism’s ability to perform oxygen-consuming physiological activities (
Killen et al. 2017). MMR has gained considerable attention across a variety of contexts in the lab and field (
Norin and Clark 2016), and could prove particularly valuable for assessments of imperilled species, which are likely to be vulnerable to the stressors associated with transportation (
Seddon et al. 2012). When organisms are released into unfamiliar environments, they must immediately confront two physiologically demanding challenges: the need to disperse in search of food resources and evading native predators (
Näslund 2021). If MMR is compromised as a result of transport stress, it could subsequently heighten the vulnerability of reintroduced fish. However, whether transport can reduce MMR, potentially increasing the underlying physiological vulnerability during reintroduction, remains unclear.
Along with finding food and evading predators, reintroduced fish must also cope with novel stressors in their unfamiliar environment, such as temperature fluctuations (
Sébastien et al. 2021). Critical thermal maximum (CT
max) is a performance metric reflecting physiological resilience that is used to assess the thermal tolerance of fishes. CT
max is the temperature at which an animal loses equilibrium, signifying an “ecological death” under natural conditions (
Lutterschmidt and Hutchison 1997;
Schulte et al. 2011). Stress can adversely affect CT
max, resulting in a decreased ability to tolerate temperature extremes or narrow the range of temperatures for fish to function optimally (e.g.,
Monirian et al. 2010;
LeBlanc et al. 2011). For example, in a study on Threadfin shad (
Dorosoma petenense), handling stress was found to significantly affect both survival and the ability to tolerate varying temperatures, resulting in higher mortality rates and lower CT
max compared to non-handled control fish (
Monirian et al. 2010). Thus, as the success of reintroduction efforts often hinges on the mitigation of stress-induced mortality and ensuring a species’ capacity to adapt to a novel environment, CT
max, like MMR, may be an important metric to consider.
The redside dace (
Clinostomus elongatus) is a small minnow species found in streams throughout northeastern North America (
Redside Dace Recovery Team 2010;
COSEWIC 2017). Populations of these minnows have significantly declined, leading to their imperilled status across much of their United States range (reviewed in
Serrao et al. 2018) and their designation as Endangered in Canada (
Redside Dace Recovery Team 2010;
COSEWIC 2017). In the wild, redside dace face many threats, including thermal stress resulting from the impact of urbanization on nearby waterbodies, compounded by the effects of climate change (
Parker et al. 1988;
Redside Dace Recovery Team 2010;
COSEWIC 2017;
Turko et al. 2020). In response to this conservation concern, captive breeding programs have been initiated in Ontario, Canada to address the decline and offer potential solutions, such as supplementing existing wild populations and providing individuals for future reintroductions. As a result, the transportation of individuals from captivity to the wild and vice versa will require appropriate techniques and strategies to ensure survival.
As reintroduction efforts for redside dace will require transport events, and neither the physiological impact of transportation stress nor potential stress-reduction strategies have been evaluated, the objective of this study is to explore the potential impacts of transport on ecologically relevant physiological traits that may be closely linked to fitness immediately after reintroduction. We hypothesize that transport will lead to changes in metabolic rate. We predict that transported redside dace will exhibit both a lower MMR and an extended period of recovery, but that high body condition fish will have higher MMR than low body condition fish, regardless of transportation. We also hypothesize that transport will reduce thermal tolerance (CTmax). We predict that as transport time increases, CTmax will decrease, but that high body condition fish will have higher CTmax than low body condition fish. Taken together, this study therefore aims to investigate the effect of a management-relevant transport event on redside dace using multiple ecologically meaningful indicators of stress, with potential future applications for species-specific reintroduction planning. This research further investigates whether employing preparatory techniques, namely, enhanced body condition through supplemental feeding, can mitigate some of the potentially negative physiological consequences of transport. More broadly, this work adds to the paucity of literature assessing transport stress in imperilled, small-bodied fishes in comparison to species with economic importance for stocking and aquaculture.
Discussion and conclusions
We found that MMR, metabolic recovery, and thermal tolerance did not differ between transported and control fish and that body condition had no effect on the response to transport across any metrics investigated. Our study represents an initial assessment of the influence of transport on redside dace physiology, and a preliminary investigation of whether thermal tolerance is altered by transport in a small-bodied fish species. Given the environmental sensitivity of redside dace and the potential for individuals to be exposed to multiple stressors in their native range (
Redside Dace Recovery Team 2010;
Turko et al. 2020), this research has implications for the recovery and reintroduction planning in this species. Our findings bode well for reintroduction efforts of redside dace, as the results of our study indicate that transport does not significantly affect the fitness-related endpoints we examined. However, wild/in-stream assessments of fitness are necessary to validate this assumption.
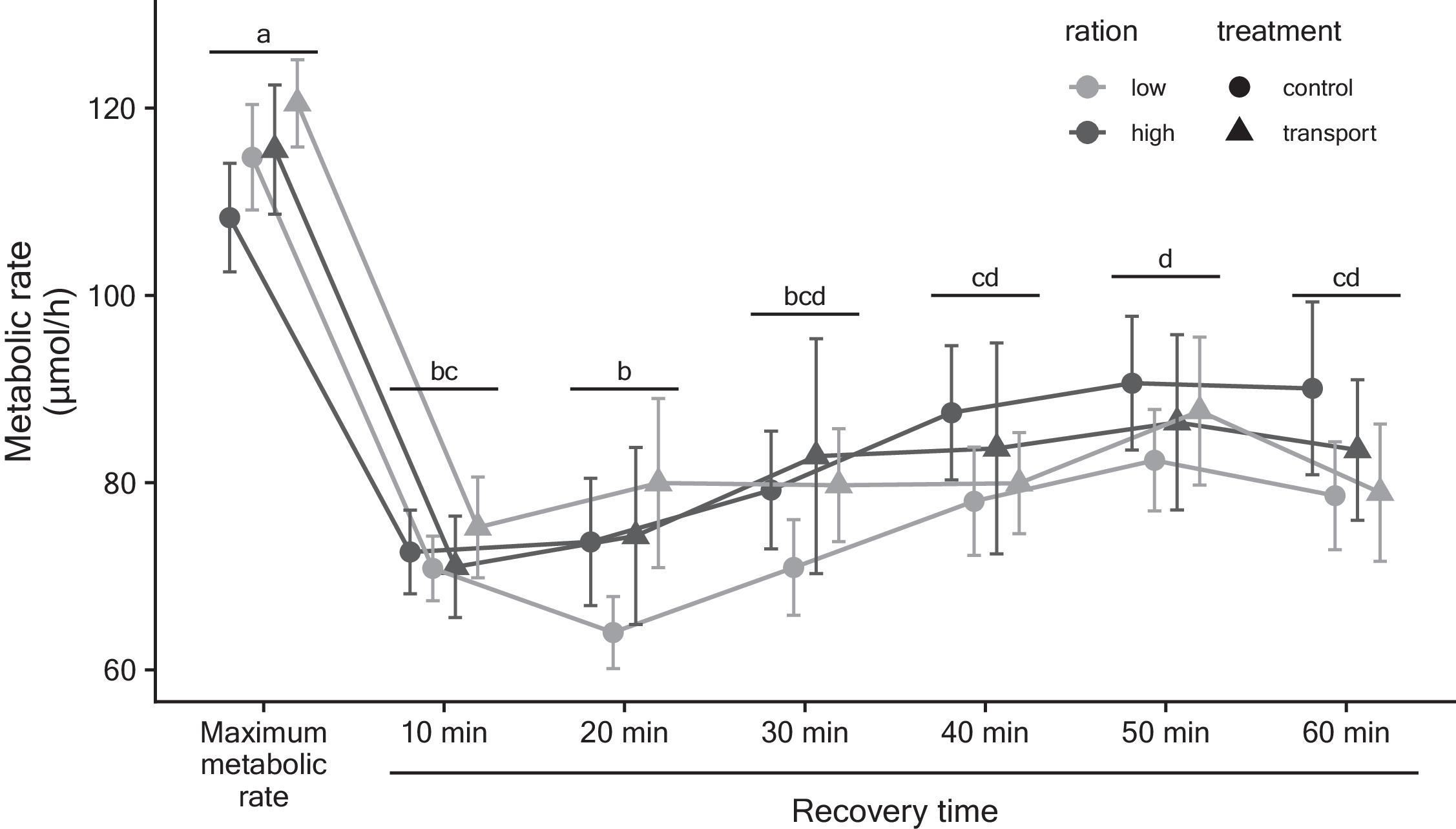
MMR was neither influenced by transport nor body condition (low vs. high ration) in our captive population of redside dace. Variation in MMR within a species has been shown to correlate with intrinsic and extrinsic factors including cardiac function, swimming performance, and the ability to flexibly respond to environmental perturbations (
Norin and Clark 2016). In addition, there is some evidence that oxygen consumption may reach values in wild fish that is close to their MMR while undertaking normal daily activities (
Murchie et al. 2011;
Norin and Clark 2016), further reinforcing the value of this physiological metric for understanding whether fish have the capacity to cope with different types of changes in their environment. In the case of our experiment, the capture, handling, and chase protocol may have been the largest contributor to MMR (
Davis and Shreck 1997), with the transport event not leading to any change or impairment to maximum aerobic capacity. Similarly, juvenile spotted grunters (
Pomodasys comersonnii) displayed the greatest metabolic response to capture and chase, with the response to additional stressors of acute temperature increase and high packing density not leading to lower MMR in comparison (
Radull et al. 2002). The transport time we employed was relatively short at 3 h and may have represented a transient demand on experimental fish. Longer transport times are routinely used in other fish species and could lead to impacts on MMR.
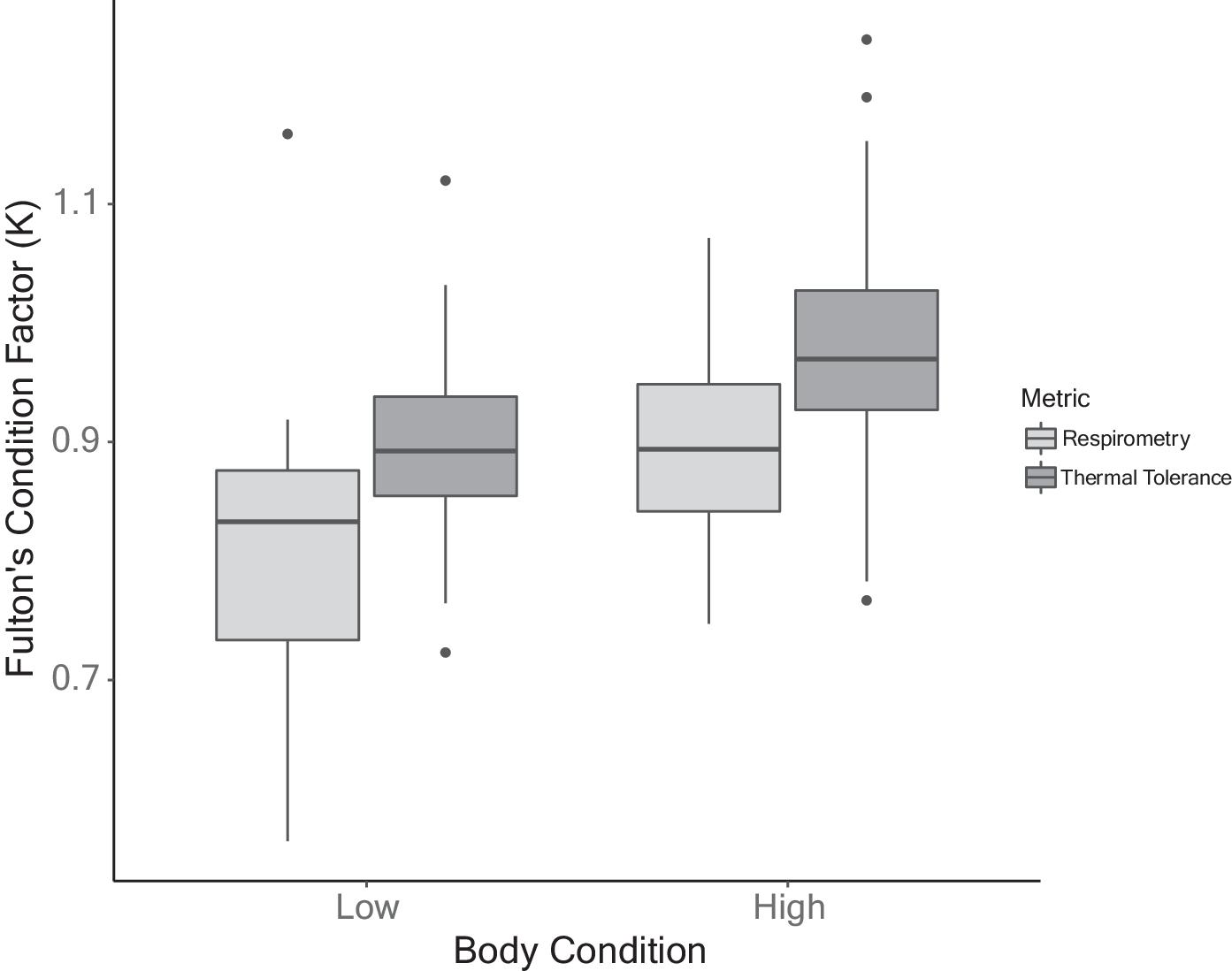
Interestingly, although the food supplementation increased body condition, it did not result in differences in MMR between supplemented and control fish. This finding is contrary to some previously reported results where improved body condition lowered MMR. For example, active metabolic rate of Atlantic Cod (
Gadus morhua L.) decreased after a 9-week feeding regime that resulted in higher condition factor compared to condition at the time of capture from the wild (
Lapointe et al. 2006). However, other studies have found results similar to ours; for instance, MMR of Brown Trout (
Salmo trutta) did not change in relation to increasing food availability (low, intermediate, ad libitum) (
Auer et al. 2016). It is possible that given, that the fish used in our experiments had been in a long-term hatchery setting and our treatments did not create a food shortage, the condition of all fish was high enough that MMR was unaffected. Indeed, it has been proposed that the predominant contributor to MMR may be skeletal muscle, which would have been unaffected by our ration treatments, and would be more likely to be affected by very limited food availability or prolonged starvation conditions (
Weibel et al. 2004;
Auer et al. 2016).
Metabolic recovery did not differ between transported and non-transported fish and was also not influenced by body condition. Under other types of stressors for fishes, recovery rate has been shown to be similar across stressor-exposed and control groups. For example,
Powell et al. (2005) found that post-exercise recovery of metabolic rate was the same in healthy Rainbow Trout compared to those infected with the microsporidium gill parasite
Loma salmonae. Further, through an indirect assessment of metabolic recovery rate using heart rate in Sockeye Salmon (
Oncorhynchus nerka), the recovery time of fish that underwent exhaustive exercise did not differ from those that underwent exhaustive exercise and were also exposed to predator alarm cue (
Lawrence et al. 2023). Given that recovery metabolism can place constraints on overall performance (
Milligan 1996) including the locomotor capacity to evade predation (
Cooke et al. 2014), our finding that the transport event did not result in an altered recovery trajectory in comparison to control fish is encouraging for post-release recovery of redside dace.
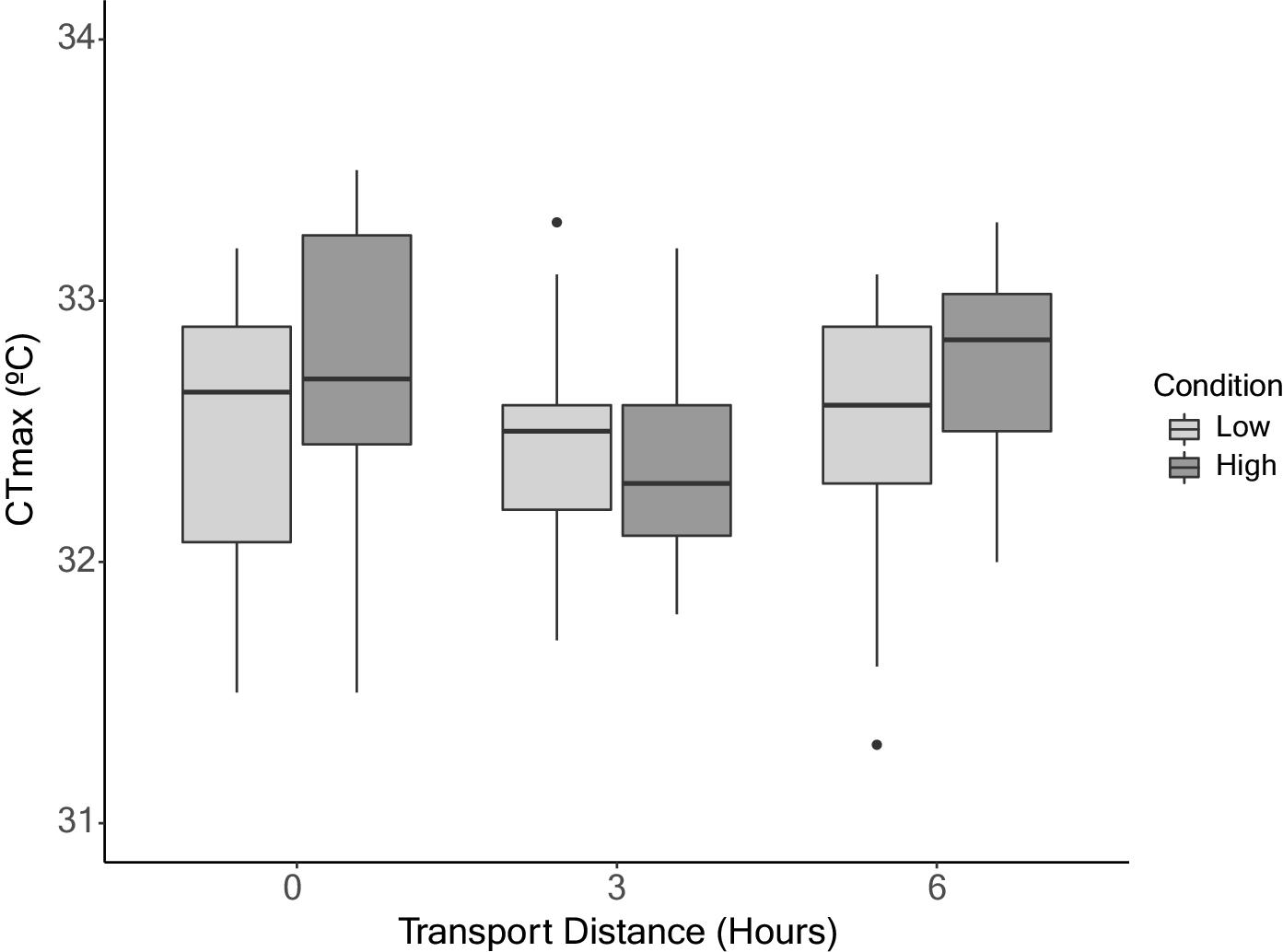
CT
max was also not influenced by transportation and body condition. While the effect of transport on CT
max in small-bodied fish remains unexplored, our findings suggest that redside dace are robust to the acute stressors associated with reintroduction. Greater body condition and/or dietary enrichment have also been associated with increased thermal tolerance in other fishes (e.g.,
Robinson et al. 2008;
Tejpal et al. 2014;
Lee et al. 2016;
Turko et al. 2020). This relationship is thought to be driven by increased energy reserves, facilitating mitochondrial function, making it possible for fish to meet the energy demands required to cope with physiological stressors, such as elevated temperatures (
Ern et al. 2023). However, contrasting findings have emerged regarding the thermal tolerance of larger individuals, which may struggle with increasing temperatures due to their surface-to-mass ratio (
Ern et al. 2023). Interestingly, our study did not uncover evidence supporting either association. Identifying a single reason for the lack of influence on CT
max by both transport and body condition remains difficult; however, our attempt to establish optimal transport conditions provides valuable insights. In this study, we aimed to mimic the priorities that would be in place during the transport and reintroduction process of an endangered species. As a result, fish were transported under low density (6 g/L) and in the climate-controlled section of a vehicle, allowing us to maintain very stable oxygen and temperature conditions. The timeframe of 3–6 h was chosen to align with likely travel distances for a reintroduction program for this species and would greatly decrease the potential for changes in water quality parameters (e.g., ammonia) (
Sampaio and Freire 2016). Altogether, these transport conditions may not have been harsh enough to elicit a marked difference in CT
max or even MMR or recovery (see above) in comparison to control (non-transported) fish. Further, the additional energetic stores of food-supplemented fish would not impart a benefit in these conditions because all fish, regardless of treatment, were in high enough condition to maintain optimal physiological functions. It is possible that during transport, food supplementation may still be a useful technique for minimizing transport stress, and we suggest that future investigations continue to employ transport times that are relevant for species-specific transport and release protocols.
Overall, our study aimed to assess the effect of transport on fitness-related metrics relevant to reintroduction, specifically MMR and CTmax. We also examined supplemental feeding to mitigate these effects. Our results show that neither transport nor body condition significantly influenced MMR, recovery, or CTmax in our captive redside dace population. These findings lend preliminary support to the viability of successfully transporting redside dace from captivity to reintroduction sites. Future research could extend this work by including fish transported from natural habitats, thereby offering insights that could guide timing decisions. While our study did not find an impact of transport on metabolism and thermal tolerance, behavioral traits, such as food intake, risk-taking behavior, orientation, and activity level, could also impact survival in a release site and should be investigated in the future. It is however encouraging that a transport event designed to simulate best-practices for this species did not result in negative effects for MMR, metabolic recovery, or thermal tolerance, given that these aspects of physiological functioning would also be important for persistence in a novel environment. In conclusion, our study provides key insight into the interplay between transportation stress, metabolism, and thermal tolerance in redside dace. Taken together, our findings underscore the feasibility of transporting redside dace to a release site and this information may possibly be extended to other small-bodied fish, for which information is lacking.