Introduction
Canada is mandated to manage the country's fisheries sustainably through implementing the Sustainable Fisheries Framework (SFF;
DFO 2009a) that supports environmentally sustainable fisheries and provides the basis for an ecosystem approach to fisheries management. A key component of the SFF is the Policy Framework on Managing Bycatch and Discards (
DFO 2013a). This policy commits Canada to manage the sustainable harvesting of aquatic species by minimizing the risk of fisheries causing serious or irreversible harm to bycatch and discard species, and accounting for total catch, including bycatch and discards. Meeting and implementing national and international commitments (e.g.,
UNCBD 2010,
2020) and policies has been incremental but Canada continues to make progress.
Understanding the impacts of commercial fisheries on the biological diversity of the ecosystem is complex. As part of the catch, nontarget catch, or bycatch, can be retained for personal use, for sale, or returned to the water, as defined by the fishing regulations (
Chopin and Arimoto 1995;
Hall et al. 2000;
Davies et al. 2009;
Gavaris et al. 2010;
Pezzack et al. 2014). Bycatch can also be characterized by the nature of the interaction with the fishery such as vessel and fishing gear interactions with marine mammals (
Read et al. 2006). There are gaps in our understanding of the magnitude of the removals and interactions of nontarget individuals due to incomplete scientific fisheries monitoring (
Davies et al. 2009;
Boudreau et al. 2017). Baseline information on the total suite of species (i.e., bycatch) implicated in some valuable commercial fisheries, such as the American lobster (
Homarus americanus) fishery in the southern Gulf of St. Lawrence (SGSL), has yet to be established.
The fisheries landscape of Atlantic Canada was previously characterized by abundant fish populations, but over the past decades the Northwest Atlantic marine ecosystem has shifted to become dominated by invertebrates (e.g.,
Frank et al. 2005;
Savenkoff et al. 2007;
Boudreau et al. 2015). Lobster supports one of the largest commercial fisheries in Atlantic Canada, an ocean region that includes the SGSL (
Fig. 1). The fishery is managed by regulating input controls, namely limiting licences, individual trap allocations, gear restrictions, fishing seasons, legal carapace sizes, and the release of egg-bearing (i.e., berried) female lobsters (
DFO 2019). Landings-based abundance indicators in the SGSL have been at a time-series high since 2012 and the stock is in the healthy zone (
DFO 2019).
Lobster is fished using a fixed gear, baited traps, which are deployed (i.e., set) from a fishing vessel and sit on the ocean floor. Lobster traps are selective by design (
Miller 1990) and constructed of wood and/or wire (
Boudreau and Giard 2022). They are built according to regulated dimensions (
Atlantic Fishery Regulations 1985b;
Boudreau and Giard 2022) and contemporary traps have also been outfitted with escapement mechanisms large enough to allow small lobsters, and other organisms, to pass through (
Rondeau et al. 2015;
DFO 2022a;
Table 1). While the gear is designed to capture lobsters, traps will occasionally catch nontarget species such as, sea urchins (
Strongylocentrotus sp.), sculpins (superfamily Cottoidea), Atlantic rock crab (
Cancer irroratus), and other mostly benthic organisms. Past studies indicate that most bycatch is nontarget lobsters (i.e., undersized or berried lobster;
den Heyer et al. 2010;
Gendron and Duluc 2012;
Pezzack et al. 2014). Lobster permit holders in the SGSL are allowed to retain male rock crabs, sculpins (
Myoxocephalus spp.), and cunners (
Tautogolabrus adspersus) for personal use (bait) or offered for commercial sale (
Atlantic Fisheries Regulations 1985a;
DFO 2009b).
The catch and discard mortality of nontarget species has generally not been of concern in the SGSL lobster fishery due to the nature of trap fishing (
Chuenpagdee et al. 2003;
Fuller et al. 2008;
Shester and Micheli 2011), the minimal diversity of bycatch, and the hand-sorting of the catch. As the nontarget species are easily sorted and quickly returned to the water, low mortality has been presumed. The discard mortality of species of conservation concern has been a focal issue in adjacent lobster fisheries. Atlantic cod (
Gadus morhua;
COSEWIC 2010), cusk (
Brosme brosme;
COSEWIC 2003, not present in the SGSL), and wolffish (
Anarchichas spp.;
COSEWIC 2012a,
2012b,
2012c) have been recorded as lobster fishery bycatch in southwest Nova Scotia (
den Heyer et al. 2010;
DFO 2013b). Canadian lobster fisheries do not have a dedicated third-party at-sea observer programme and the recording of bycatch in the vessel logbook is considered unreliable. The insufficient collection of bycatch data in the SGSL lobster fisheries has become of more importance as the fishery is certified as sustainable by the Marine Stewardship Council (MSC;
Criquet et al. 2014,
2015). In the MSC assessment reports, conditions were set by MSC to provide more bycatch information from the SGSL fisheries to demonstrate minimal impacts on the ecosystem (
Criquet et al. 2014,
2015).
While the survival of discarded (i.e., returned to the water) nontarget catch from the lobster fishery has been assumed to be high, time spent captive in the trap and confined with other lobsters, exposure to the elements once the trap is at the surface, and handling could cause injury or otherwise stress the organism. Discard mortality rates are challenging to obtain, but qualitative methods have been developed to assess the potential of survival of species by recording injuries and monitoring vitality prerelease (e.g.,
Benoît et al. 2010,
2011,
2012). Accounting for all sources of fisheries mortality, including the nontarget catch, can better inform stock assessment and science advice. For example, when Atlantic cod discarded from the Gulf of Maine (USA) lobster fishery were included in the cod stock assessment, diagnostics and stock trajectories for cod were improved (
Boenish and Chen 2020).
To begin to examine ecosystem impacts of the lobster fishery through the lens of bycatch species, the diversity captured, relative amount, and discard mortality, the present study has two main objectives. First is to describe the bycatch of the SGSL spring and summer lobster fisheries. Second is to adapt and operationalize a semiquantitative injury and vitality scoring system (per
Benoît et al. 2010,
2012) to describe injuries and assess the vitality of species caught as bycatch. For the purpose of this study, bycatch will be defined as all organisms caught in the lobster trap that are a nonharvestable (or nontarget) lobster or a species or taxonomic group other than lobster. As Canada builds towards an ecosystem-based approach and in support of sustainable fisheries management, specific to minimizing the impacts and risk of mortality to species caught incidentally and discarded in the SGSL lobster fishery, we present results to support continued and more frequent catch monitoring.
Methods
Sampling protocol and design
Sampling locations were selected in partnership with industry collaborators, the Maritime Fishermen's Union and the Prince Edward Island (PEI) Fishermen's Association. To ensure representative spatial coverage of the SGSL lobster fishery, the participants fished in the Lobster Fishing Areas (LFAs) where they are licenced and on their typical fishing grounds; however, this also depended on participant availability. The research activities were authorized by a scientific licence issued by Fisheries and Oceans Canada under the s. 52 of the Fishery General Regulations. The selected areas (
Fig. 1) were sampled with the same participating fishers almost every week throughout the 2015 fishing seasons; the spring fishery operated from April 30 to June 30 in LFAs 23A–D, 24, and 26A, and the summer fishery in LFA 25 was from August 9 to October 10. Sampling began during the second week of the fishing seasons. All traps hauled (see trap allocations in
Table 1) were to be sampled for bycatch.
Scientific personnel were deployed to monitor and record fishing activities on the lobster vessels. For every sampled trip, information of gear used and fishing practices were collected including daily weather and air temperature at the first and last trap hauled. Bottom temperature was recorded every 2 h by a VEMCO© Minilog data logger installed on a fishing trap at each site.
Bycatch composition, injury, and vitality
All nontarget organisms captured either in or on outside the traps, were considered bycatch; specimens were counted and weighed. Rock crabs were separated by sex as males can legally be retained by lobster licence holders, while females are required to be returned to water. We note that a minimum legal size (MLS) for male rock crab retained as bycatch was introduced in 2021 (
DFO 2021). The sculpin species (
Myoxocephalus spp
.) can also be legally retained and were subsequently grouped together. Some invertebrates were recorded at a higher taxonomic level, e.g., hermit crab (
Pagurus spp.), sea star (
Asterias spp.), and periwinkles (
Littorina spp.), to streamline the sampling. All lobsters caught were measured (carapace length, CL; in mm) and sexed, but not weighed; lobsters that could not be legally retained (smaller than the MLS, window or maximum size females, and berried females) were returned to water after being sampled. The weights of discarded lobster were calculated using length–weight relationships described by
Rondeau et al. (2015;
eqs. 1 and
2).
Undersized (i.e., sublegal) lobsters were assessed for vitality on an ad hoc basis, giving the priority to the other species. This was because of the presumed high survival rate out of water for lobsters given the success of the live-animal market that has also been documented (
Lavallee et al. 2000;
Lorenzon et al. 2007). Berried female lobsters were not assessed for vitality and were immediately returned to the water after assessing for injury to limit air exposure and potential egg losses.
When categorizing female lobsters, size regulations were prioritized before the presence of eggs, i.e., undersized females with eggs were categorized as undersized first. The different MLS for lobster per area and other size restrictions are presented in
Table 1. The data are being analyzed using the 2015 size regulations; however, the MLS has been increasing incrementally across the LFAs at various paces.
Nearly all specimens, except the lobsters as noted above, caught as bycatch were assessed using predetermined criteria to score for injury (
Tables 2 and
3) and vitality (condition;
Table 4) immediately upon being taken out of the traps. Exceptions include taxa for which it was impractical to do an assessment (e.g., jellyfish,
Cyanea capillata; barnacles, Cirripedia) and the subsampling of abundant species (e.g., rock crabs, cunners, sea urchins;
Strongylocentrotus droebachiensis). Species assessed at any level of conservation concern by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) were sampled as per the study protocols. The exception was Striped wolffish (
Anarhichas lupus, also known as the Atlantic wolffish), which are protected under the
Species at Risk Act (SARA) (
COSEWIC 2012a) and if captured were sampled for weight and length but not retained for further assessments and quickly returned to the water. Other specimens from various taxa were not fully assessed if they were left in the trap and out of the water for more than 1 min. For example, if a fish was found in the trap after the sample was collected, it was counted as bycatch but not assessed for vitality.
The vitality assessment was designed based on
Benoît et al.’s (2010,
2012) semiquantitative assessment and began as soon as the trap came out of the water and the animals were removed. Specimens were individually observed for 10 consecutive minutes to reflect the maximum time that would elapse before organisms would be sorted and returned to the water during the fishery. During the 10 min observation period, the organisms were placed in separate containers, assessed for recent injuries that would have occurred in the trap, and monitored every minute for changes in vitality. Injury and vitality assessments performed were based on scores designed for fish (
Benoît et al. 2010,
2012;
Tables 2 and
4). In the present study, the assessments were extended to include invertebrates and the scoring was modified accordingly (
Tables 3 and
4). Before being returned to the water, fish (fork length rounding up to nearest cm, except for Atlantic herring;
Clupea harrengus, pinched length rounding down to nearest 0.5 mm) and crabs (carapace width rounding down to nearest mm) were measured as per regular sampling protocols, weighed, and air temperature was also recorded. Because spring scales were used to measure individual weights, the movement of the vessel may have introduced errors into overall weights.
Given the wide array of bycatch species that could have been encountered during the lobster fishery, samplers were provided with more precise signs to look for when assessing vitality (
Table 4). Fish species were easier to assess by looking at breathing attempts (movements of the mouth and/or operculum) and body movements as a response to gentle stimuli. For crustaceans, such as undersized lobsters and crabs, body and mouthparts tonus and movements were a sign of good vitality as well as the retraction of eyes when touched. Crabs tended to nestle into a corner when out of the water but eye movements and legs contraction were deemed indicators of good vitality. Various vitality signs were assessed for echinoderms; in sea urchins and sea stars, samplers were looking for movements of tube feet and spines, if the specimens were adhering to a flat surface (i.e., the container), and if mouthparts were tightly shut. For gastropods and bivalves, if the operculum or the valves were tightly shut, the animal was considered to have good vitality. Gastropods moving around the sampling container were also deemed to be in good condition.
Exposure time
To assess the amount of time bycatch species were typically exposed to the air between the time that traps were hauled from the water and subsequently sorted, still-frame (one frame per second) cameras were installed on the fishing boats participating in the study. Recordings were then examined to calculate the exposure or sorting time. The exposure time began from the moment the trap was out of the water and continued until the trap was emptied of all organisms. Traps were sorted by the fishing crew as per daily fishing operations, typical sorting speed, and the catch was handled as it normally would. Sorting time was not recorded for traps not fully visible on the video. Median sorting time per fishery (spring and summer) was calculated using individual trap sorting times.
Data treatment
Data were summarized separately for the spring and summer fishing seasons as they reflect different temperature regimes, both for air and bottom temperatures. Similarly, fishing locations differ between seasons, which could potentially affect bycatch composition, quantity and survival potential. Catches (kg) per trip, with standard error (SE), of bycatch groupings (unharvestable lobsters per regulations, legally retained bycatch species, other fish, other crustaceans, and invertebrates) were calculated by LFA as bycatch was not recorded trap by trap. The sum of the traps sampled per trip was recorded. For differences between two means, homogeneity of variances was tested using Levene's test, followed by two-sample t tests.
Average daily bottom temperatures were calculated at 1400 hours (spring) or 1800 hours (summer) the day before the haul and 0400 hours the day of the haul to represent when the trap was actually fishing and to avoid false air temperature data when traps are being hauled during the day. Fishing activities are spread over a longer period during the summer fishery providing the rationale for the later starting time (1800 hours) for the temperature calculation.
Bycatch in 2019
During the 2019 lobster fishing seasons, a repeat sampling for bycatch was undertaken by the Maritime Fishermen's Union and the PEI Fishermen's Association in LFAs 23B, 23D, 24, and 26A in spring and LFA 25 in summer. This study employed the same sampling procedures to measure and weigh bycatch; however, no vitality, injury, temperature, or trap handling times were measured. The spring fishing season took place from April 30 to June 30 in LFA 23B and D, and April 29 to June 29 in LFA 24 and 26A. In LFA 25, the fishing season was from August 8 to October 9. These data were summarized to support the abundance and composition of bycatch in the SGSL lobster fisheries. Lobster were categorized per 2019 legal size regulations (Table S1). Trips with an incomplete record of samples were excluded from the summaries.
Results
Spring sampling (LFAs 23A–D, 24, 26A) began May 11 and ran through July 2, 2015 (
Table 5). Thirteen captains participated and data were gathered from 51 fishing trips and a total of 14 348 traps were sampled. Sampling during the summer fishery (LFA 25) took place between August 13 and October 7, 2015, and 4989 traps were sampled during 22 fishing trips (
Table 5). Trap soak time was either 1 or 2 days (
Table 5). The majority of the trips (46 of 73; 63%) used mackerel (
Scomber scombrus) to bait their traps, with occasional use of Gaspereau (
Alosa pseudoharengus; 9), Atlantic herring (8), redfish (
Sebastes spp.; 4), flounder (Pleuronectiformes unspecified; 4), hake (
Urophycis spp.; 1), and Atlantic silversides (
Menidia menidia; 1). Including lobster, a total of 28 species and taxonomic groups were recorded during the sampling activities, 15 invertebrate, and 13 fish (
Table 6). All species caught in or on the lobster trap were recorded, including those that would not have actively entered the trap. Catch information was pooled by trip, LFA, and season, but not by trap.
Bycatch composition
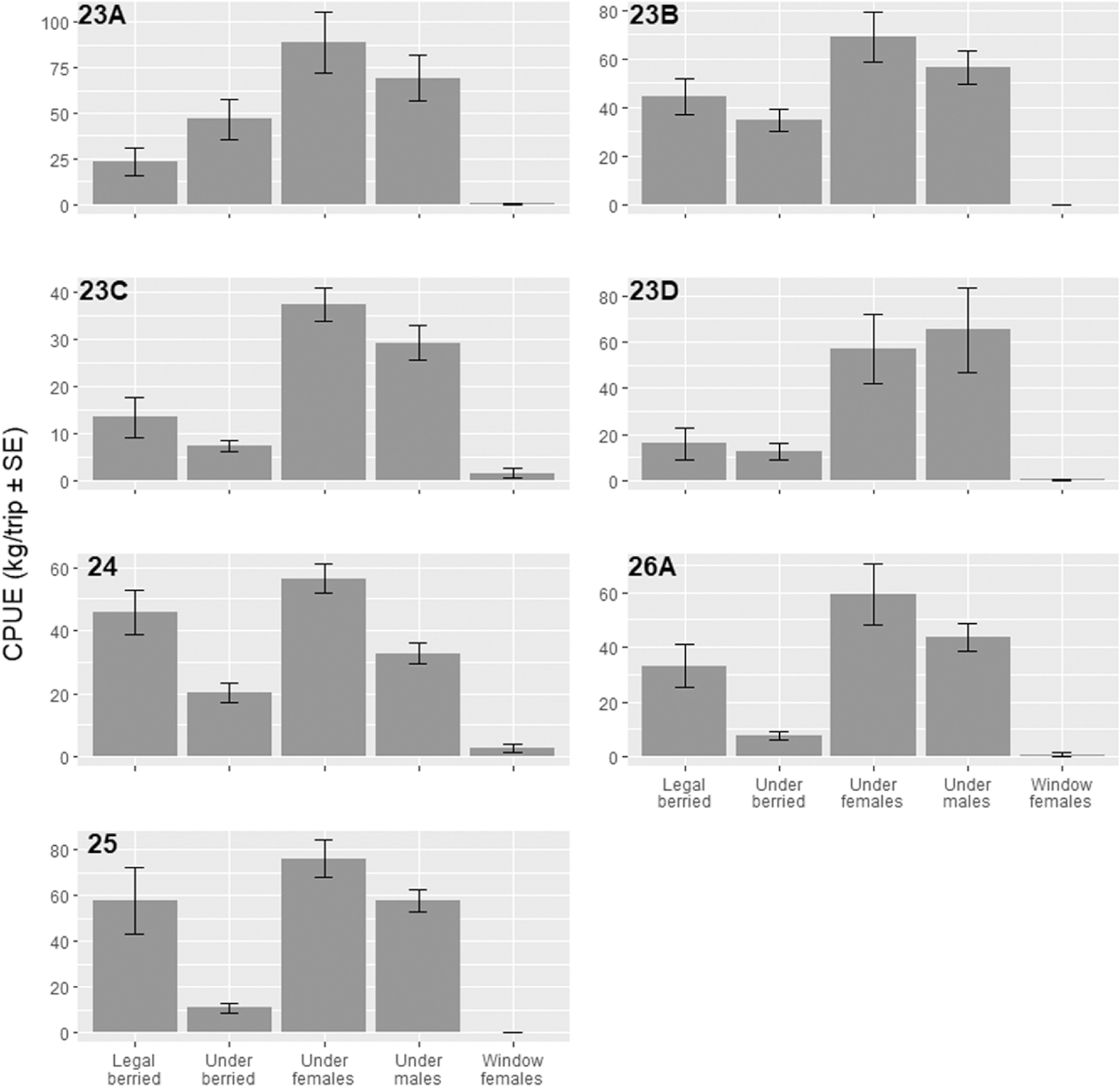
A total of 32 456 individual organisms weighing a total of 9044.83 kg were recorded during the spring fishing season, and 19 493 organisms weighing 4942.87 kg were sampled in the summer (
Table 6). The majority of bycatch was nontarget lobster, i.e., the nonharvestable lobster per the fishing regulations, over 80% by number and weight in both the spring and summer fisheries, with over two-thirds of the discarded lobster catch being females (undersized, berried, window/maximum size combined) (
Table 6). Undersized lobsters comprised 71% and 63% of bycatch by weight in the spring and the summer fishery, respectively (
Table 6). These trends are also reflected by weight per trip by LFA (Table S2,
Fig. 2), with all undersized lobsters (male and female) being the most abundant bycatch (kg) per trip in all LFAs. During the spring season, the catch of undersized females ranged from 88.76 kg/trip (±17.03 SE) in LFA 23 A to 37.41 kg/trip (±3.48 SE) in LFA 23C, and catching 76.07 kg/trip (±8.12 SE) in the summer (Table S2;
Fig. 2). Berried lobster made up 30% and 31% by weight of the bycatch in the spring and summer fishery, respectively (
Table 6). The catches of berried females (legal-sized) in spring ranged from 45.81 kg/trip (±6.87 SE) in LFA 24 to 13.39 kg/trip (±4.30 SE) in LFA 23C, while the summer catch per unit effort (CPUE) was 57.84 (±14.31 SE) (Table S2;
Fig. 2). Though the most captured nonharvestable lobsters were females (berried and undersized), notably few large or window-sized females were captured in this study (
Tables 6 and S2;
Fig. 2).
Other than lobster, 27 different taxa groups were recorded, 23 in the spring fishery and 16 in the summer (
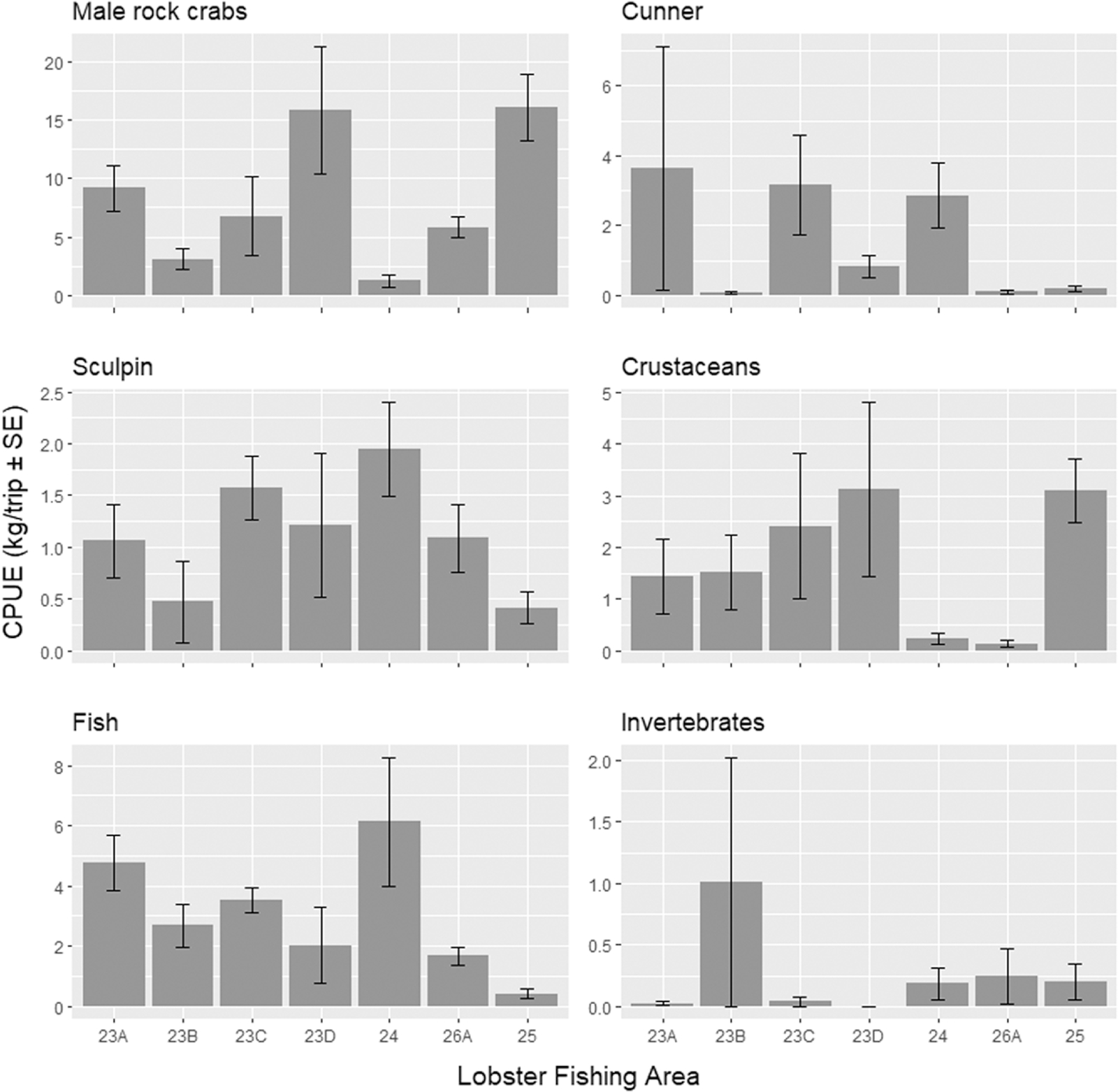
Table 6). Rock crab (male and female) were the next most abundant species of bycatch observed by weight (
Tables 6 and S3;
Fig. 3), comprising 4% of total bycatch including the nontarget lobsters, and 49% excluding nontarget lobsters, in the spring, and 8% with nontarget lobsters, and 88% without, in the summer fishery. Cunner was next most abundant species in the spring fishery (13% by weight of the nonlobster bycatch), while in the summer fishery hermit crab (
Pagurus spp.) was the next most abundant species (5% by weight of the nonlobster bycatch). All remaining bycatch species recorded in both seasons represented less than 1% of the remaining composition (with and without lobster).
Lobster licence holders in the SGSL are authorized to keep male rock crab, cunner and sculpin caught in their lobster traps for personal use or sale; however, it is not known whether those captured as part of this study would have been retained or discarded during regular fishing activities. When considering the average weight of bycatch captured per fishing trip (CPUE kg/trip) for the legally retained bycatch species cunner, the CPUE varied by LFA ranging from 0.06 (±0.04 SE) in LFA 23B to 3.65 (±3.48 SE) in LFA 23 A in the spring, and 0.20 (±0.08 SE) in the summer in LFA 25 (Table S3;
Fig. 3). For sculpin, CPUE ranged from 0.48 (±0.39 SE) in LFA 23B to 1.95 (±0.46 SE) in LFA 24 in the spring, and 0.42 (±0.16 SE) in LFA 25 (Table S3;
Fig. 3). Male rock crabs had the highest CPUE ranging from 1.23 (±0.57 SE) in LFA 24 and 15.83 (±5.43 SE) in LFA 23D in the spring, and 16.07 (±2.83 SE) in the summer (Table S3;
Fig. 3).
There were species of conservation concern recorded (
Table 6; Fig. S1). One specimen of striped wolffish, protected under SARA, was captured during the first sampling trip of the spring fishery (LFA 24) and was measured and weighed then immediately released and not retained for vitality assessment. Four species assessed as conservation concerns by COSEWIC—Atlantic cod, white hake, and winter skate (all Endangered;
COSEWIC 2010,
2013,
2015), and American plaice (Threatened;
COSEWIC 2009)—were also recorded (
Table 6) and assessed for vitality (Table S4) as per the protocol. All 20 Atlantic cod were captured in a single sampling area (LFA 24), as were the six American plaice (LFA 26A). The unique white hake was caught in LFA 23C and the winter skate in LFA 25. One specimen from the endemic population of lady crab (
Voutier and Hanson 2008) was captured during the summer fishery. There are some potential trends to monitor, for example cod was captured exclusively in LFA 24, to the north of PEI, and mostly early in the season, in mean bottom temperatures below 10 °C (Fig. S1).
Bycatch in 2019
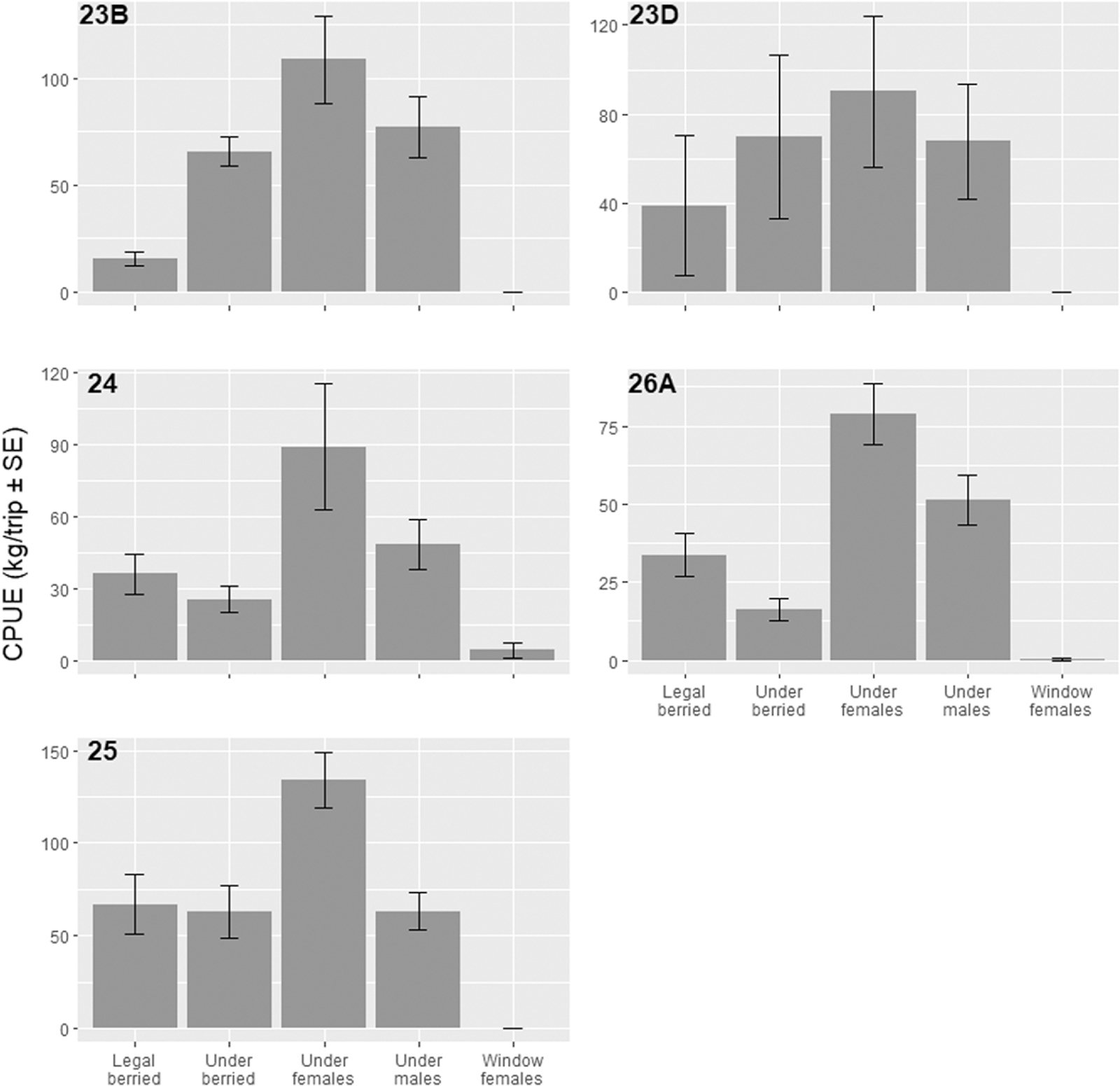
In 2019, a total of 30 trips took place, 22 during the spring (6 in LFA 23B, 4 in 23D, 8 in 24, and 4 in 26A) and 8 during the summer (LFA 25). One trip in LFA 23B and one in 23D had incomplete sampling records and were removed from the dataset. The remaining 28 trips were summarized and are presented. Trips in the spring took place from May 8, 2019 to June 28, 2019 and in the summer from August 14, 2019 to October 1, 2019 and more than 6068 traps were sampled (1500 in 23B, unknown in 23D, 2058 in 24, 1047 in 26A, and >1463 in 25). Soak days were recorded for three LFAs and ranged from 1 to 3 days with the median of 1 day (average of 1.17 days ± 0.17 SE in LFA 24, 1.33 ± 0.33 in LFA 26 A, and 1.67 ± 0.67 in LFA 25). Nontarget lobsters were the most abundant bycatch by weight per LFA and per trip (Tables S5 and S6;
Fig. 4). Undersized females were the most captured per trip in the spring ranging from 108.80 kg/trip (±20.40 SE) in LFA 23B to 78.65 kg/trip (±9.70 SE) in LFA 26A; in the summer, the CPUE was 133.97 kg/trip (±14.86 SE) (Table S6;
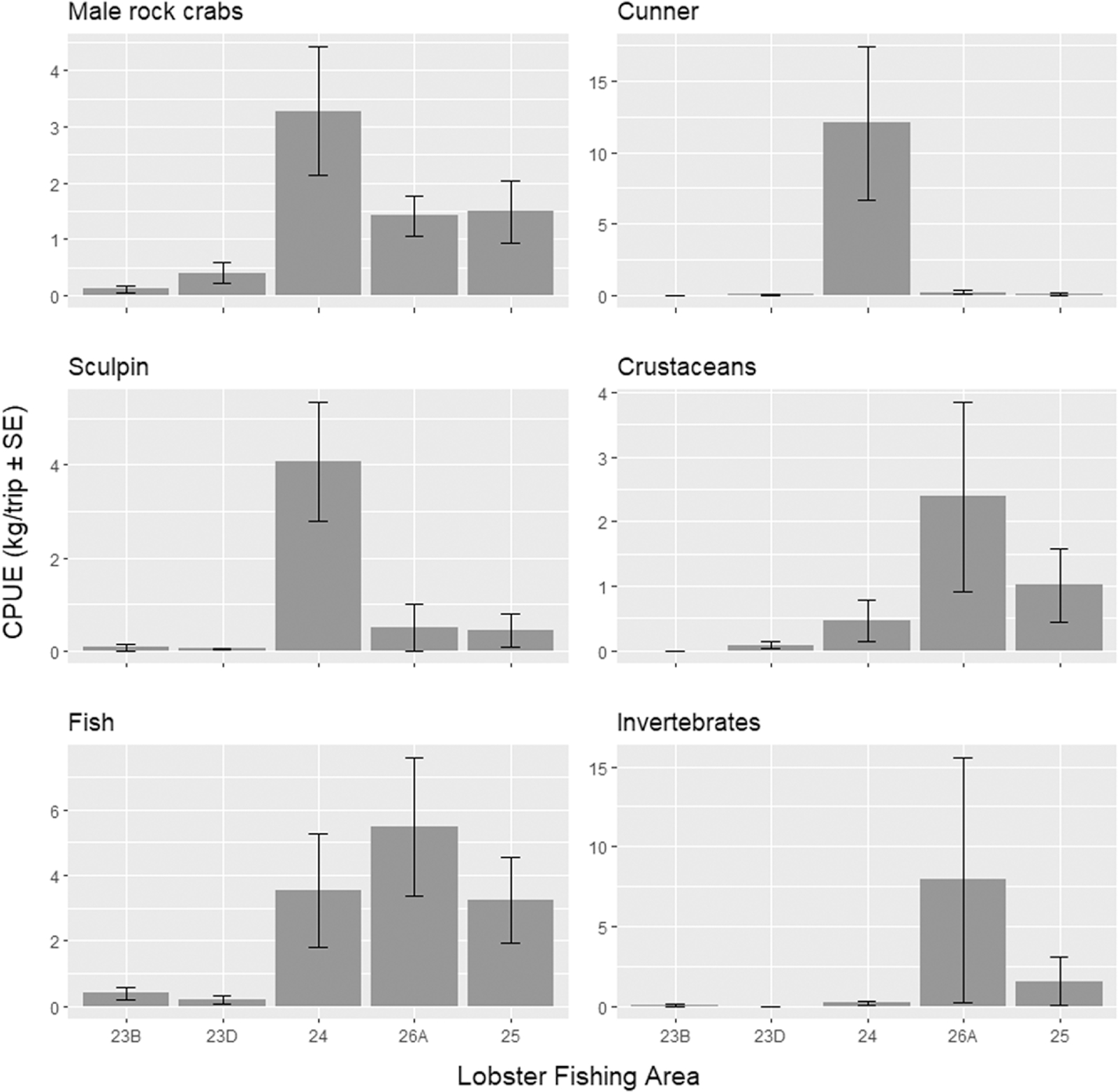
Fig. 4). A total of 841 nonlobster bycatch specimens weighing 63.05 kg were sampled in summer and 837 weighing 266.20 kg in spring (Table S5). The spring fishery recorded 22 different taxa groups, while summer captured 13 (Table S5). The species with the highest catch rate in spring were cunner (12.05 kg/trip ± 5.37 SE) in LFA 24 followed by invertebrates in LFA 26A (7.92 kg/trip ± 7.67 SE), and nonretainable fish were highest in the summer (3.25 kg/trip ± 1.31 SE) (Table S7;
Fig. 5). Additional species recorded were snow crab (
Chionoectes opilio) and Atlantic lumpfish (
Cyclopterus lumpus, Threatened;
COSEWIC 2017) during the spring fishery (Table S5). In addition to the lumpfish, Atlantic cod was the other species of concern sampled, with one cod in the spring in LFA 24, consistent with 2015, and an additional two recorded in the summer in LFA 25 (Table S5).
Exposure time and temperature
The time to process each lobster trap ranged from a few seconds to several minutes (
Table 7). Twenty of 51 trips in spring and 13 of 22 in summer did not have a video recorded (Tables S8 and S9). Median sorting times for a trap for each LFA (representing air exposure time for bycatch) in the spring (
N = 7896) and summer (
N = 1467) fisheries were under 1 min (
Table 7) ranging from a median of 12 to 43 s across the LFAs participating in the spring fishery, and 20 to 45 s in the summer (
Table 7). The seasonal mean soak days were not statistically different from each other (Welch's two-sample
t test, spring
, summer
,
t = −1.58, df = 23.68,
p value = 0.13). Generally the mean bottom temperature at the beginning of the season was cooler than at the end, with the mean range of the spring fisheries being between 1.23 and 14.68 °C, and 9.35 and 20.28 °C in summer (
Table 7, S8 and S9). Mean bottom temperatures were statistically different between the two seasons (two-sample
t test, spring
, summer
,
t = −9.34, df = 67,
p value < 0.05). With respect to air temperatures, the spring is cooler, though occasionally reaching as high as 28.5 °C (
Tables 7, S8, and S9). The temperature (°C) taken at the last sample of the day, when the air should be at its warmest, was significantly different between the seasons (two-sample
t test, spring
, summer
,
t = −5.41, df = 74,
p value < 0.05).
Injury and vitality
There were 3500 invertebrates and 1057 fish individually (4557 total) assessed for injury and vitality (Table S4). Of the invertebrates assessed, the majority were rock crab (
N = 2005) and lobster (
N = 1188). There was no observed mortality of invertebrates or fish; however, minor and major injuries were observed (Table S4). Five invertebrates—three rock crabs and two lobster—had major injuries (0.1% of invertebrates, injury code 3; see
Table 3) (Table S4) and 96 invertebrates—1 sea star, 59 rock crab, and 36 lobster—had minor injuries (1.8% of invertebrates, injury code 2; see
Table 3). Of the fish assessed for injury, 15 individuals—6 cunner, 3 Greenland cod, 1 longhorn sculpin, 2 shorthorn sculpins, and 3 winter flounder (1.4% of fish)—had a minor injury (code 2; see
Table 2), and the remainder were assessed by the protocol to be uninjured (97.6% of invertebrates, 98.6% of fish; Table S4).
With respect to vitality, virtually no variation in invertebrate observations occurred during the 10 min observation period (Table S4; Figs. S2–S4), with the exception of a rock crab whose vitality code (
Table 4) switched from 3 (poor) to 4 (moribund or dead) after 10 min (spring season, major shell break with exposed organs and no chance of survival; Table S4; Fig. S3C), and a sea star (spring, injury 2, vitality assessment from 1 to 3 over the 10 min; Table S4; Fig. S4B). There was no mortality during the vitality observations for invertebrates, though there were some assessed to be moribund (code 4). For the fish, one cunner was moribund (spring, injury 1, changing from vitality 1 to 4 in 10 min); a poor condition (code 3) at the end of observation was recorded for one Greenland cod (summer) and three winter flounder (two in spring, one in summer) (Table S4; Fig. S5). Our exploratory analyses did not yield significant relationships in the present study, e.g., linking injury, vitality (alive vs. moribund), temperature, season, and exposure time (per
Benoît et al. 2012) due to the high survivorship.
As an observation regarding postrelease survival, predation events were witnessed by the samplers on species being returned to the water after observation. For example, sea ravens have a swim bladder that became inflated over the 10 min, and while they were exhibiting vital signs, they were floating at the surface when released and were preyed upon by sea birds.
Discussion
To support Canada's commitment to sustainable fisheries and contribute to our knowledge of the impact of lobster fisheries on the ecosystem, a systematic study was conducted to establish a baseline of incidental catch during the SGSL lobster fishing seasons. Given the importance of the fishery to the region, the high abundance of lobster, and the role of bycatch as a driver of overexploitation and a barrier to species recovery (
McDevitt-Irwinn et al. 2015), understanding the cumulative impact of lobster fishing effort on the benthic community is necessary. Here, we have thoroughly described the composition of the incidental catch, both nontarget lobster and nonlobster species, over the 2015 fishing seasons (spring and summer) in the SGSL, with the exception of LFA 26B. While the SGSL fishery caught a wide range of taxa, the quantities observed were fewer than anticipated (
Table 6; see also Table S5 for 2019). Our findings are consistent with other Atlantic Canadian lobster fisheries where the most common nontarget catch were undersized lobsters in addition to rock crab (
Tables 6 and S5;
Figs. 2 and
4;
Gendron and Duluc 2012;
Pezzack et al. 2014).
The hypothesis that the impact on bycatch from a trap fishery would be low due to short exposure times and the rapid return of nontarget catch to the water was supported by the processing time, temperature observations, and associated injury and vitality scores. The fishers were skilled at sorting the traps, with the median handling time being 45 s or less for both the spring and summer fisheries (
Table 7). In the SGSL, lobster occupy a wide range of environmental conditions with the majority of densities being found in autumn bottom water temperatures between 2.5 and 18.2 °C and as expected the bottom temperatures in this study fell within the known habitat indices (
Chassé et al. 2014). While lobsters are able to survive up to 3 days out of water in cool and moist conditions, warmer temperatures (∼15 °C) are more physiologically stressful (
Lorenzon et al. 2007). Catches were exposed to a wide range of air temperatures occasionally nearing 30 °C and the specimens likely benefitted from the rapid processing of the traps.
To measure how proxies for exposure such as sorting time and temperature impact bycatch condition, semiquantitative assessments of injury and vitality were employed for fish and invertebrates (
Tables 2–
4;
Benoît et al. 2010,
2012). The methodology of assessing the vitality of fish prior to release has been demonstrated as a good predictor of mortality (e.g.,
Richards et al. 1995;
Benoît et al. 2010). The majority of the individuals assessed for injury in the present study (∼98% both fish and invertebrates) were deemed uninjured. It is also possible that as the lobster population and correlating density within the traps increase, higher injury rates will follow. A field note from the present study reported that specimens would require to be pried out of lobsters' claws during sorting on occasion; this would injure fish in particular. Nearly all individuals were assessed to be alive throughout the observations of vitality before being returned to the water (Table S4). There is some nuance to this assessment, however, as delayed mortality is possible (e.g.,
Davis and Olla 2001;
Yochum et al. 2017,
2018;
Anderson et al. 2020), and predation events were observed on returned catch that were experiencing physiological or behavioural stress (as also observed in
Anderson et al. 2020).
Hauling, handling, and air exposure did not cause immediate mortality as evaluated from the visual assessments of injury and vitality. It is possible that postrelease there is mortality due to prospective barotrauma, predation, or other impacts, though the good condition of specimens prerelease is encouraging. We acknowledge that due to limited monitoring activities it is challenging to observe trends in capture for rare bycatch species or bycatch caught in very small numbers, which can introduce difficultly in detecting trends in species of interest or concern (
Table 6; Fig. S1). There are multiple factors and traits that can influence an organism's likelihood of survival; however, fish with swim bladders are the most at risk for postrelease mortality (
Benoît et al. 2010,
2011,
2012,
2013). Species of conservation concern with swim bladders in the present study, Atlantic cod and White hake, were assessed to be uninjured and to have excellent or good condition after 10 min (Table S4, Fig. S5). The survival of cod is the most tenuous (
Benoît et al. 2010,
2012), with 65% survival of SGSL cod (vitality code of 1—excellent) observed 48 h postcapture from fishing (
Benoît et al. 2010); however, the shallow nature of the lobster trap fishery (typically <50 m depth;
Boudreau and Giard 2022) is likely to lower the risk of postrelease mortality (≥40% mortality observed from recreational angling from >50 m depth;
Ferter et al. 2015). In the Gulf of Maine lobster fishery, Atlantic cod discard mortality was estimated to be 24.8% (
Sweezey et al. 2020). There are other factors that influence the resilience of a fish species to handling and exposure, for example those that are more sedentary and with a higher tolerance to hypoxia (e.g., sculpins, sea raven, skates) have been estimated to have low discard mortality (
Benoît et al. 2013).
For invertebrate taxa, as new methodologies and protocols are put into practice, there is potential to continue to adapt the injury and vitality assessments for future bycatch studies. In the present study, the high rankings of vitality for nearly all observations (Table S4), the survival of the incidental catch prerelease was assessed to be excellent per the protocols and low mortality postrelease was anticipated (as in
Benoît 2011). There is evidence from other fisheries of low discard mortality of decapods, for example, the immediate probability of survival for undersized discarded Mediterranean spiny lobster (
Palinurus elephas) was estimated to be high (0.64) in a trammel net fishery (
Catanese et al. 2018). In a Dungeness crab (
Cancer magister) trap fishery (Oregon, USA), discard mortality rates for male and female crabs (5 days postrelease) were estimated to be <0.1 using a reflex action mortality predictor approach (
Yochum et al. 2017). Many of the invertebrate species recorded (
Table 6) are intertidal organisms that would be tolerant to some exposure.
There is also the possibility of cumulative impacts of being recaptured throughout a season. For example, undersized female lobster or egg-bearing females are arguably the most vulnerable life-stage of lobster bycatch and it is assumed that the act of being caught likely increases the risk of injury, or mortality, egg loss, and predation. The recapture rates of discarded lobsters in tagging studies indicates there is good survivorship post handling and release (e.g.,
Tremblay and Eagles 1997;
Comeau and Savoie 2002). The handling of nontarget lobster in general, however, is expected to be high over the course of a season, despite escapement mechanisms in the trap to encourage their exit. While the size of escape mechanisms have been increasing alongside MLS (
Rondeau et al. 2015), incorporating more than those required could further minimize bycatch and reduce sorting time. As sampling took place during typical fishing operations, the present study did not standardized the size or number of escapement mechanism on the traps. The minimum regulated dimensions of the entrance and escapes (
Table 1) were identical, or varied by 1 mm, between LFAs. Fishers have also reported to install larger-than-regulated escapement mechanisms into their trap design to further reduce trap sorting and bycatch (
Boudreau and Giard 2022). The next iteration of this study would benefit from being able to examine the correlation between bycatch, trap design (e.g., standardizing the size and number of escapement mechanisms;
DFO 2022a), additional biological data (e.g., shell hardness, specific injuries, i.e., missing claws), and fishery catch location. Catch, and bycatch, may be better linked to traps and trap location rather than scaled to landings because benthic organisms are often patchy in distribution, or have the choice to enter the trap. Sampling a larger proportion of the fishing grounds would provide important data on how well the presented results and trends represent the fishery as a whole.
A time series of fisheries-independent data can be used to develop abundance indices for monitoring and assessment including the species of conservation concern and others. Here, we present a CPUE index (kg/trip for 2015 and 2019) that could be considered an early baseline for species captured in the lobster fishery (see
Zhang and Chen 2015;
Boenish and Chen 2020). The level of acceptable discard mortality risk for species of conservation concern may vary depending on the lens being applied. In the case of the SGSL Atlantic cod stock (
Swain et al. 2019), evidence from this study and others (
Sweezey et al. 2020) indicates that the lobster fishery is not a likely major source of mortality. However, unaccounted-for mortality of bycatch species may impact recovery plans for species at risk or of critically low abundance. A new scientific monitoring programme would collect data to assess changes in bycatch for all species caught in the lobster fishery, including those of concern, low abundance, or data deficient, such as striped wolffish, American plaice, and lady crab.
There are some upcoming challenges for species caught by the lobster fishery, particularly for those used as bait. The critically low Gulf of St. Lawrence spring Atlantic herring and mackerel fisheries were closed in 2022 (
DFO 2022b), which is anticipated to create issues with sourcing bait in the upcoming season(s) (
Calder et al. 2022). This indicates that there may be increased pressure on species that are legally retained for use as bait, namely cunner, male rock crab, and sculpin (
Myoxocephalus spp. were grouped in this study) where there is limited science and data. Without proper monitoring, it will be difficult to understand any implications or changes in usage. Rock crabs are considered to be a key prey item to support lobster growth and reproduction (
Gendron et al. 2001), and are an important species in the SGSL food web (
Hanson et al. 2014). It will be important to take a full account of their total fisheries mortality including direct rock crab fisheries in addition to bycatch in the lobster fishery, as male rock crabs are the most dominant legal bycatch. As a conservation measure, an MLS regulation was implemented for male rock crab in the lobster fishery in 2021 (
DFO 2021). The present study can serve as a baseline for these retainable species, future monitoring is recommended in concert as the industry adapts to status of traditional bait availability and forage fish fisheries sustainability.
More recently, the fishery has been assessed as one to avoid by the
Monterey Bay Aquarium (2022) Seafood Watch Program due to risks of entanglement of North Atlantic right whales (
Eubalaena glacialis) in fishing gear, and also cautions around the use of herring as a bait source. Improved monitoring of all sources of anthropogenic mortality are encouraged to better encompass all of the different facets of sustainable fisheries management.