Introduction
Animals living in risky environments can manage the risk by at least four inter-related mechanisms (
Brown and Kotler 2004): time allocation (honing activity periods to times of low risk), space use (choosing habitats and foraging patches where risk is low, e.g.,
Creel et al. 2005), apprehension (reducing risk by being aware or vigilant), and foraging tenacity (maintaining harvest rates in the presence of danger). To these we can add social benefits of grouping behaviours and cooperation. The allocation of each mechanism will depend on such characteristics as the temporal pattern of risk (
Lima and Bednekoff 1999;
Higginson et al. 2012), the forager’s state (
McArthur et al. 2014;
Monclús et al. 2015;
Bannister and Morris 2016), the density and spatial distribution of competing individuals (
China et al. 2008;
Dupuch et al. 2014), trade-offs between resource quality and risk (
Lima and Dill 1990;
McArthur et al. 2014), the functional and numerical responses of predators (
Dupuch et al. 2014), and the scale of environmental variation (e.g.,
Druce et al. 2006;
Heithaus and Dill 2006;
Hodson et al. 2010). The joint characteristics of predators and prey and their interaction with spatial variation in habitat thus dictate the prey species’ landscape of fear—their spatially explicit map of predation risk (
Brown et al. 1999;
Altendorf et al. 2001;
Laundré et al. 2001;
van der Merwe and Brown 2008;
Laundré et al. 2010). Although the literature on fearful foraging is extensive, we do not generally know the conditions under which foragers favour one strategy over another or how those strategies might vary with spatial scale.
Advancing our understanding of risk management requires an evaluation of each mechanism, and its potential interactions with others, in a natural and spatially variable environment. We thus designed experiments on snowshoe hares (Lepus americanus Erxleben, 1777) to explore how spatial variation influences their risk management strategies and to determine how habitat can modulate behavioural mechanisms that reduce predation risk.
We set the stage by describing our study system and its appropriateness for assessing risk management. Next, we link risk with concepts of fine and coarse-grained foraging. We use those concepts to generate a priori expectations enabling risk management by hares. We then describe three experiments that we used to test each expected outcome. We analyze each experiment separately and synthesize the results to yield a complete picture of hares’ perception of, and behavioural responses to, spatial variation in predation risk. We conclude with a short discussion of the insights that snowshoe hare foraging provides to our understanding of risk management.
Snowshoe hare study system
Snowshoe hares are an appropriate model system for studies of risk management for at least four inter-dependent reasons. Hares seek refuge when not foraging, they forage singly, they are deeply embedded as a keystone species in climate-induced predator–prey cycles with Canada lynx (
Lynx canadensis Kerr, 1792) (
Krebs et al. 2001a,
2001b;
Krebs 2011;
Yan et al. 2013), and they occupy a stressful but time-dependent landscape of fear (
Boonstra et al. 1998;
Sheriff et al. 2009;
Krebs et al. 2014). Hares in fragmented or patchy landscapes are especially vulnerable to predation (
Wolff 1980;
Wirsing et al. 2002), and virtually all hares meet death at the teeth and talons of their predators (
Krebs et al. 2014). Hares reduce risk in winter by time allocation. They forage from dusk through dawn and tend to avoid open areas during moonlit nights (
Gilbert and Boutin 1991). Little is known, however, about the ways in which hares might integrate other mechanisms into a spatially dependent risk-management strategy.
With these points in mind, we chose two sites located within alder (
Alnus viridis (Chaix) DC.) dominated shrublands on a 40 ha area regenerating from agriculture and forest harvest in northwestern Ontario, Canada (48°19′N, 89°47′W). Alder habitat is preferred by snowshoe hares in winter because it shelters animals from the elements and provides refuge from predators (
Pietz and Tester 1983).
Site 1 represented fine grain where there was a sharp ecotone between dense 3 m tall alder and an abandoned, open, 2.6–4.8 m wide logging trail. Site 2 represented coarse grain where large patches (>30 m wide) of open grassland–old field with sparsely distributed 3 m tall red pine (
Pinus resinosa Aiton) saplings were embedded along a similarly sharp ecotone within the alder matrix (
Morris 2005). Snowshoe hares consumed the few shrubs and saplings within less dense areas of alder, the more numerous shrubs along the ecotone toward more open habitat, and lower branches of pine in otherwise open areas. Hares did not browse the mature alder. Hares tend to avoid open habitat when possible and forage there with less intensity than in adjacent forest habitat (
Morris 2005;
Hodson et al. 2010) presumably because they perceive a greater risk of predation (
Keith et al. 1984;
Smith et al. 1988;
Hik 1995;
Rohner and Krebs 1996).
Hares and their heavily used runways were abundant in both sites. Runways crossed over the linear logging trail (yielding fine-grained use along runways) but not the larger open-field patches (coarse grain) where their sporadic tracks were scattered towards individual foraging (e.g., red pine) opportunities. We verified that the hares lived in a fearful landscape by documenting the presence of predator species including lynx, red fox (Vulpes vulpes fulvus (Desmarest, 1820)), coyote (Canis latrans Say, 1823), grey wolf (Canis lupus Linnaeus, 1758), fisher (Martes pennanti (Erxleben, 1777)), pine marten (Martes americana (Turton, 1806)), American mink (Neovison vison (Schreber, 1777)), and several large owl species (via direct observation, wildlife camera photos during our experiments at the same stations used for observing hares, or spoor). Crepuscular great gray owls (Strix nebulosa Forster, 1772) posed a significant risk to hares near dawn and dusk, whereas more nocturnal horned owls (Bubo virginianus (Gmelin, 1788)) and barred owls (Strix varia Barton, 1799) also hunted hares overnight. Wing marks in snow, blood, and body parts confirmed that two hares were killed by avian predators at our study transects immediately after the experiments concluded (one each at the fine-grained and coarse-grained scale, both at the boundary between open and alder habitats).
Risk management in heterogeneous environments
We can gain insight into the tools available for managing predation risk by contemplating the interdependence between each mechanism and the spatial scale of resource patches (
Brown 1999). Scale will depend on the spatial acuity and movement capacity of individuals, as well as constraints imposed by spatial patterns in the foraging landscape.
We can formalize our insights by contemplating the optimal harvest rate expected from prey individuals exposed to risk. We follow
Gilliam and Fraser (1987) by considering a forager that can choose to remain in a safe refuge or forage in two habitats that differ in risk. We further assume that the forager maximizes its survival subject to procuring some minimal amount of resource necessary to maintain its energetic state (e.g., snowshoe hares surviving winter). Under these conditions,
where QHR is quitting-harvest rate, the instantaneous foraging rate obtainable when the last forager leaves the resource patch;
C is the metabolic cost of foraging;
μ is the instantaneous likelihood of being killed by a predator while in the patch;
P is the probability of surviving the foraging period;
ϕt is the marginal fitness of engaging in alternative activities (including time spent in the refuge);
ϕF is the marginal value of survival (the only direct component of fitness for non-reproducing individuals); and
∂F/∂E is the marginal value of fitness in terms of energy (
Brown 1992).
Imagine that the foraging species occupies a patchy environment where its movement characteristics cause individuals to encounter habitat and resource patches in the proportions in which they occur (a fine-grained environment,
Levins 1962). Individuals can nevertheless alter their space use by allocating more time to safe habitat and foraging patches than they do to risky ones. Their ability to do so will increase as the scale of the environment moves towards coarse grain (defined as the scale where individuals have the option to occupy one habitat only,
Levins 1962; e.g., use only the safe habitat and its resource patches). We thus expect, if individuals manage risk by time allocation, that hares will prefer the safe alder habitat over risky open areas and that their preference for alder will be greater at the coarse-grained scale.
Vigilance reduces risk, so if hares manage risk with vigilance, we expect higher vigilance in risky as opposed to safe habitat. If risk is related to cover, then vigilance should be reduced in experiments that supplement safety with cover.
Exposure to risk at the fine-grained scale is directly proportional to the frequency of the two habitats. The potential to preferentially use safe habitat is increased at coarse scales, and thus mean vigilance might be reduced. But if risk increases with the size of risky patches, then hares should be more vigilant in risky habitat at the coarse-grained scale than at the fine-grained scale.
Environmental grain will also influence foraging tenacity. To predict the outcome, assume that time spent vigilant reduces risk, is traded off against active foraging time, and that the quitting-harvest rate, for a given level of resource, decreases as the amount of time spent in the foraging patch increases. The expected quitting-harvest rates (
eq. (1)) will be equal between equally accessible safe and risky places, but not the density of the resource when the final forager leaves the patch (giving-up density (GUD),
Brown 1988). Vigilant foragers will thus leave risky patches at a higher GUD because they have allocated less of their available foraging time to resource harvest.
Each mechanism can, in theory, respond to experiments that enhance the safety of foraging patches through additional cover. If, for example, hares are less vigilant at foraging sites provisioned with supplemental cover, then the GUD should be lower at sites supplied with additional cover. Finally, if risk in open areas increases with distance from safety, then vigilance in the open habitat should increase and GUD should also increase with distance from alder.
Knowing these expectations, we designed experiments to assess foraging and vigilance in identical resource patches located at different scales in relatively safe versus risky habitats. We did so by taking advantage of the repeated travel along snow-packed runways by snowshoe hares in winter. We contrasted foraging between risky open spaces and the safe refuge afforded by thick alder-covered habitats (
Pietz and Tester 1983). Several other studies confirm that hare survival is higher in and near to densely vegetated habitats than in habitats with sparse cover (e.g.,
Hodson et al. 2010;
Feierabend and Kielland 2015).
We were able to include the spatial scale of safety by locating sampling stations along runways that either crossed (fine-grained exposure; runways crossing the logging trail) or did not cross (coarse grain; a few tracks but no packed runways in old-field openings) the two habitats. We then alternated safe versus risky foraging opportunities by adding supplemental cover to resource patches in the riskier open habitat. We designed each experiment such that the supplemental cover in risky habitat mimicked the normal safety that hares would experience in the safety of alder. The design did not allow us to test hypotheses on the effects of additional safety in both habitats.
Hares are thus the tool we use to test theory predicting the effects of scale on the strategies that prey use to manage their perceived risks of predation. We emphasize that our tests of scale refer only to strategies hares might employ when faced with fine versus coarse-grained foraging alternatives. Our research was not designed to evaluate how those strategies might change from one landscape or population to another. Our emphasis is on strategies, not the behaviour of individual hares or their population dynamics. Most studies on snowshoe hares, including those that contemplate space use (
Wolff 1980;
Wirsing et al. 2002), predation risk (
Keith et al. 1984;
Smith et al. 1988;
Hik 1995) and state-dependent foraging (e.g.,
Murray 2002) are dedicated towards their use as a model for understanding population cycles (
Krebs et al. 2001a,
2001b;
Preisser 2009;
Krebs 2011, and references therein). Although risk management is applicable to cyclical dynamics, hares at any point in a cycle should adopt strategies of risk management that maximize fitness. We assess predictions emerging from that assumption.
In summary, hares might manage risk by the following expectations. We aim to determine which subset of these strategies they actually use, and they are either confirmed or rejected in the Results section.
1.
Hares should prefer the ostensibly safe alder habitat over an open habitat.
2.
Hares should spend more time in alder when patches of open habitat are large (coarse-grained scale) than when they are small (fine-grained scale).
3.
Hares should be more vigilant in open habitat than in alder habitat.
4.
Hares should be less vigilant under supplemental cover.
5.
Hares should be more vigilant in risky habitats at the coarse-grained scale.
6.
Hares should forage more tenaciously (lower GUD) at sites with supplemental cover in an otherwise risky open habitat.
7.
Vigilance in open areas should increase with distance from safety.
8.
GUDs in open areas should increase with increasing distance from safe (alder) habitat.
Materials and methods
Experimental design
We conducted three experiments on free-ranging hares during winter from 2010 to 2011. Each experiment provisioned foraging patches with two 50 cm long premeasured juvenile jack pine (Pinus banksiana Lamb) boughs. We uniquely marked each bough with a small label (orange flagging tape) secured (with a thumbtack) to the smoothly cut base. The labels allowed us to unambiguously assess the stem diameter at point of browse for each bough.
Jack pine is a highly preferred winter food source for hares (
Bergeron and Tardif 1988), and hares readily consume jack pine boughs. Nutritional value declines from the tip of the bough to the base (
Palo et al. 1992;
Hodson et al. 2010), hares prefer the distal end, so the marginal benefit of foraging declines as hares consume a greater proportion of the bough. Stem diameter at point of browse thus yields a reliable and repeatable estimate of hares’ GUD (
Morris 2005;
Hodson et al. 2010). We collected boughs from ∼10-year-old regenerating jack pine stands, excluded any boughs with cones, measured the basal diameter of the remaining boughs with a digital caliper, and retained only those with a diameter between 7.0 and 10.5 mm. These diameters guaranteed reduced browse quality towards the base of the stems (
Palo et al. 1992;
Morris 2005;
Hodson et al. 2010) that is necessary to meet the GUD assumption of diminishing returns within foraging patches.
We located study transects at active hare runways and imbedded randomly chosen pairs of boughs vertically in the snowpack to a depth of approximately 5 cm. The orange labels were completely obscured at the base of each bough. We monitored hare behaviour and activity by mounting time-synchronized motion detecting wildlife cameras (Reconyx RapidFire PC90, Homen, Wisconsin, USA) on t-bar metal posts at the boundary between alder and open habitat. Night-time images were recorded with an infra-red illuminator, but the camera’s operation is undetectable by hares. The two time-synchronized cameras were programmed to shoot single images, with identical fields of view and a 5 s lag between consecutive images at each study transect. Cameras were arranged back-to-back such that one camera recorded animals in the open while the other one recorded animals in alder. We evaluated the hares’ management of predation risk by contrasting still images of hares and their GUD between the two habitats. Cameras operated continuously throughout the day and night but virtually all hare activity was either crepuscular or nocturnal.
We complemented the observational data with treatments that manipulated predation risk by adding cover over boughs in open habitat. Cover consisted of a “tepee” framed from 10 freshly cut 3 m long alder stems with profuse side branches. We provide illustrations of the experiments and hare behaviour online (
Photographs S1–S6;
Fig. S1).
Experiments were initiated following a one-night trial. Hares actively foraged the trial boughs on each transect. We chose not to increase the length of the trial period because (1) it was obvious that the hares found and consumed the boughs, (2) we did not want to risk habituating the hares to the food source, (3) we did not want to alter densities by attracting new hares to the foraging sites, and (4) our cross-over design that alternated treatments by day (below) eliminated any short-term temporal pattern within experiments.
We wore large snowshoes during all fieldwork to minimize disturbance to the snowpack. All experiments and procedures followed the guidelines for research on wild mammals established by the American Society of Mammalogists (
Sikes 2016). The research was conducted under the authority of an approved animal care protocol (#07 07-08) issued by Lakehead University’s Animal Care Committee (ACC). The ACC follows all applicable institutional and national guidelines for the care and use of animals.
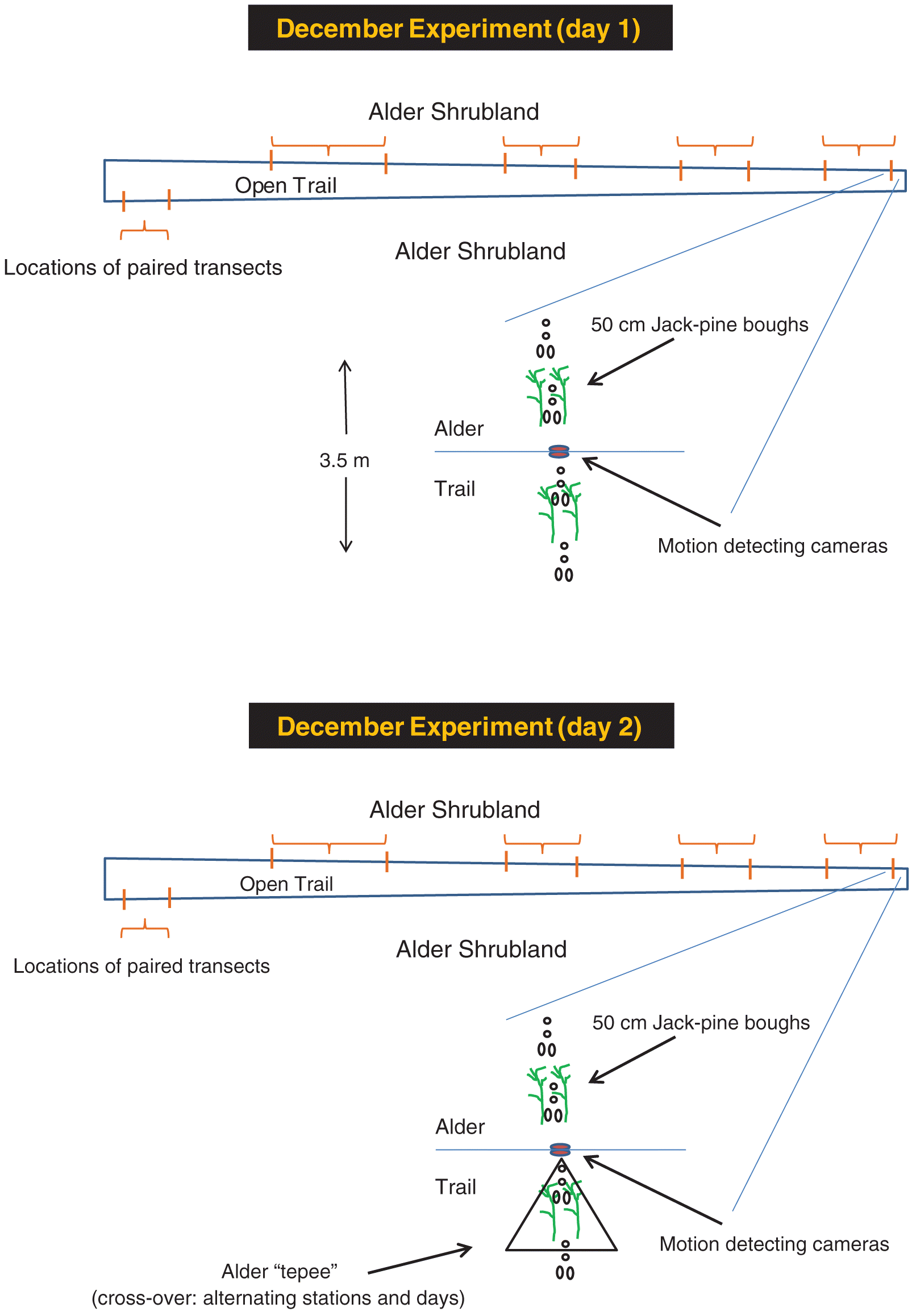
Experiment 1 (December 2010): Fine grain
We established 10 different 3.5 m long mini-transects at hare runways connecting alder shrubland on opposite sides of open habitat along the abandoned logging trail (
Fig. 1;
Photographs S1–S2). The design thus included 10 foraging stations, each comprised of two pine boughs, in each habitat (40 boughs each on two different days) with 20 back-to-back cameras. There was no vehicle traffic, use by other humans, or snow clearing. Transects were separated from one another by at least 20 m.
We randomly selected alternating transects as control (no tepee) or cover (open boughs placed centrally inside a tepee) for each of two experimental nights (22 and 23 December 2010). We placed a pair of randomly chosen boughs at 1.73 m distance from the sharp ecotone separately in open (in the logging trail) and alder habitat during daylight hours on 21 December 2010. The distance corresponded with a 2 m hypotenuse from the 1 m high camera and thus guaranteed equal fields of view for both cameras. We collected and measured the diameter at point of browse on the foraged boughs on 22 December when we switched control and cover treatments (by moving the tepees to the adjacent paired control transect). We placed another 40 boughs along the 10 transects as before, collected and measured them on 23 December, and downloaded the camera images the following day.
We examined each hare image and classified the hare as vigilant (alert and erect or standing posture, eyes and ears directed away from pine boughs,
Photographs S3–S6) or not. Animals traveling along the runways from one habitat to the other yielded partial images in the camera’s field of view. We included these images in the not vigilant category. The numbers of such images were similar in each habitat (467 in alder and 406 in open). We confirmed that their inclusion had no effect on our analyses by removing them and re-assessing the proportions of vigilant versus non-vigilant images. We did not consider images with two or more hares (1.7% of all images) as representing vigilance. We estimated the role of vigilance in the hares’ management of predation risk from the frequency of vigilant behaviours in each habitat and treatment separately for each transect. We repeated these assessments in experiments 2 and 3.
Experiment 2 (January 2011): Coarse grain
We established four new 20 m long transects two weeks later at the ecotone between alder shrubland and open-field habitats on 8 January 2011 (
Fig. S1). No other large-scale sites were available to increase sample size for this experiment. Snow conditions and ambient temperatures were similar to those of experiment 1. We mounted the cameras slightly above snowpack to capture distant images (again with equal fields of view). We placed a pair of pine boughs at the ecotone and 10 additional pairs along each transect at 2 m intervals extending into both habitats (five pairs in each habitat) on 9 January. We collected the boughs on 10 January then placed only one pair of boughs at the ecotone to collect control data on the hares’ use of the two habitats in the absence of additional resources. We collected and measured all foraged boughs and downloaded camera images on 11 January.
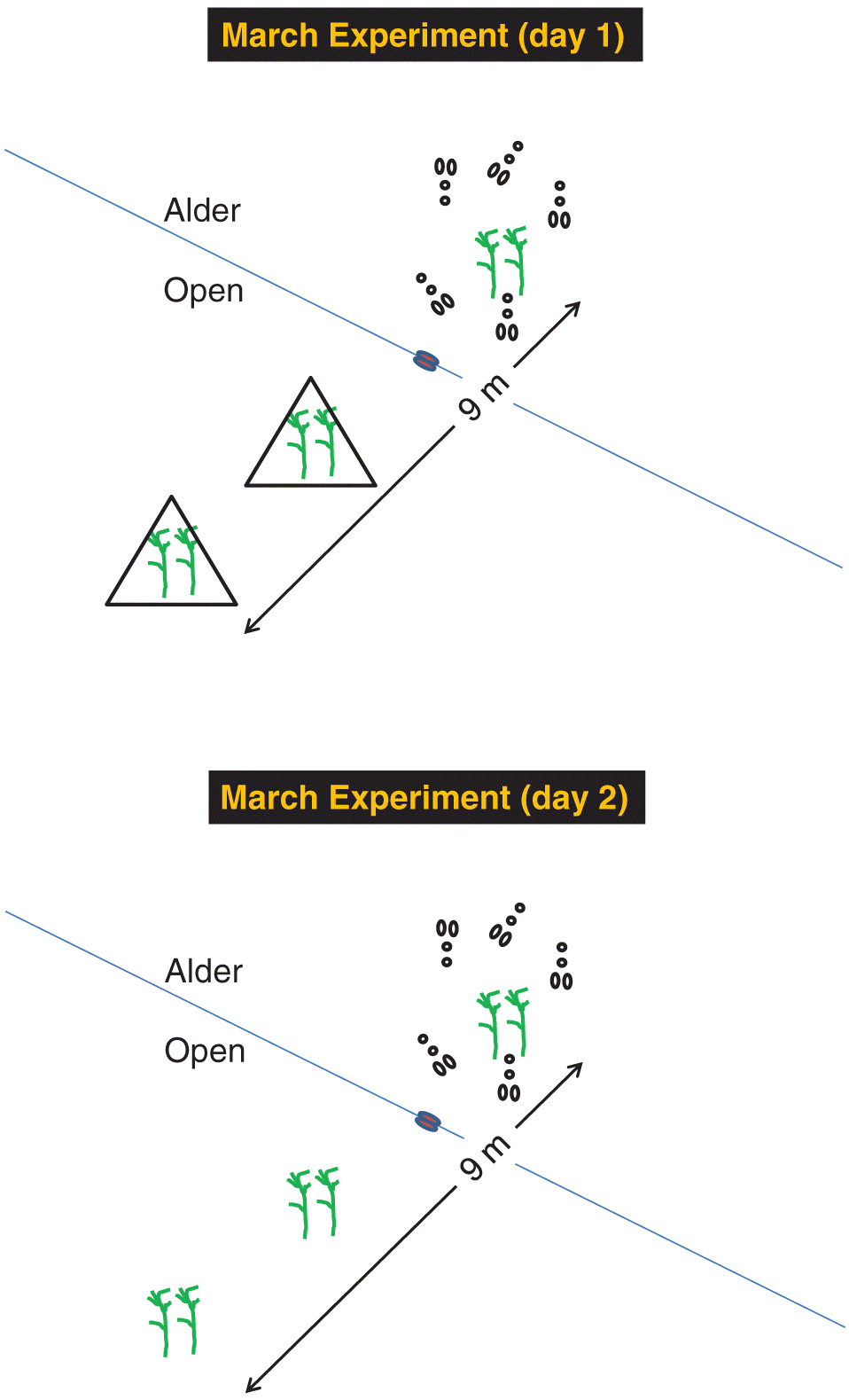
Experiment 3 (March 2011): Coarse grain
Experiments 1 and 2 did not allow us to test for the effects of supplemental cover at the two different scales so we conducted a third experiment in early March 2011 when we assumed that carry-over effects from experiment 2 were dissipated. The experiment used the same transect and camera locations as experiment 2 but with a different placement of pine boughs (
Fig. 2). We placed one pair of boughs in the alder at 3 m past the ecotone and two additional pairs in the open at 3 m and 6 m from the ecotone on 6 March. We randomly chose two of the four transects as controls (no tepees in the open) and two as treatments (alder tepees placed over both sets of boughs in open habitat). We collected and measured foraged boughs on 7 March, moved the tepees to the other two transects, and placed new boughs at each station. We collected and measured these additional control boughs on 8 March, moved the tepees back to the original transects, repeated the entire cross-over design on 9 and 10 March, and downloaded images the following day.
We cannot rule out the possibility of temporal changes, such as environmental conditions (clear sky changing to overcast and light snow in January; overcast and light snow changing to partly sunny and light snow in March), that may have occurred since we completed experiment 2. Please note, however, that even with such changes, they would affect only the experimental addition of cover, not our January test of spatial scale.
Data analysis
Our design was based on paired data. Knowing this, we evaluated the relative use of alder versus open habitat with paired t tests contrasting the total number of camera images observed at each transect. These data are unbiased by habitat (equal fields of view). We used similar paired t tests to evaluate the number of images with and without tepees and those that were vigilant versus non-vigilant (asin*sqrt transform of the proportion of images displaying vigilance, coarse grain only). Pairing our data by habitat eliminates the error variance between treatments and thus yields a more powerful test than would have been possible with unpaired data. Even so, our tests at the coarse-grained scale are based on small samples, so our interpretations assume that effects of small and variable magnitude are ineffective in managing predation risk.
We searched for differences in vigilance behaviour in experiment 1 (fine grain) with a saturated repeated measures (observations on different days) mixed model of the arcsine transformed (asin*sqrt) proportion of vigilant images (fixed effects = habitat (alder versus logging trail), treatment (tepee versus no tepee), and day (day 1 or 2 of the cross-over design), random effect = transect). We used a similar repeated measures mixed model to assess differences in GUD (mean diameter of the two stems at their point of browse) between habitats, treatments, and days while controlling for covariates of the proportion of vigilant images (asin*sqrt transform) and mean basal stem diameter (experiment 1) and between the fixed effects of habitat and distance from the ecotone (experiment 2, same random effect and covariates). We were concerned that the mixed model for mean browse diameters in experiment 1 was too complicated for the size of our data set. So we confirmed its results with a second test by calculating the mean browse diameter in each habitat for each transect and treatment. We then tabulated the ratio of the mean browse diameter in alder divided by that in open for each pair of observations. Ratios >1 would document that the mean GUD on a single transect and day was greater than the paired value in the open habitat. Ratios <1 would document that the mean GUD in alder was less than in the open.
We analyzed differences in vigilance behaviour at the course-grained scale (experiments 2 and 3) by tabulating the proportion of vigilant versus non-vigilant (asin*sqrt transform) images at each transect in each habitat. We were concerned that snowfall on 7 March 2011 may have compromised the cross-over design for foraging tenacity (a significant difference among days), so we corrected the problem by calculating the mean GUD for the two treatments (tepee versus no tepee) for each transect and day at each distance (standardizes observations by transect and day and thus eliminates the influence of weather). We converted these data into a binary variable (= 0 if the GUD at 6 m > GUD at 3 m; = 1 otherwise) and contrasted the totals to assess the interaction between distance from cover and treatment with these paired data. We calculated two similar binary variables representing whether mean browse diameter and the proportion of vigilant images at each station in the field were greater or smaller when the station was covered by a tepee or open (no tepee). We used a single-classification goodness-of-fit test to evaluate the treatment effect and similarly to assess differences between alder and open habitats of a cumulated binary variable contrasting GUD at only the 3 m distance (0 if GUD in open > GUD in alder; 1 otherwise; there were no pine boughs at 6 m in the alder habitat).
Moon phase varied between experiments 1 and 2 and it is possible that resource access, and possibly hare demography, also varied and altered the state of foraging hares. We reasoned that changes in state (
Houston and McNamara 1999) would be reflected in significant differences in GUDs (state alters the marginal value of fitness in terms of energy,
eq. (1)). We tested for such an effect with a mixed model (dependent variable = browse diameter; covariate = basal diameter; random = transect) comparing data between experiment 1 and the first pair of pine boughs in experiment 2. The analysis contrasted only foraging at stations lacking additional cover (tepees were used in experiment 1 but not in experiment 2). All analyses were conducted with SPSS (Version 22, IBM Analytics, International Business Machines Corp., Armonk, New York, USA) and Minitab (Version 17, Minitab Inc., State College, Pennsylvania, USA) software.
Some readers may wonder whether the linear scale of our experiments (∼1 km) was too small. Perhaps only one or two hares foraged in our experiments. The reported short-term home-range sizes of snowshoe hares (radius ∼0.1 km (3 ha), e.g.,
Boutin 1984, fig. 6;
Wolff 1980, fig. 8; and
Feierabend and Kielland 2014, size of mean core areas ∼0.4 to 0.8 ha) strongly suggest that the scale or our design was indeed appropriate. Regardless, we begin the results with a test of this concern by counting the numbers of hares that our synchronized cameras recorded during each consecutive minute on each transect (the maximum number observed by either camera on a single transect, but not by both). We reasoned that hares would not move from one transect to another within the 1 min observation period so this number represents a conservative estimate of the number of simultaneously active snowshoe hares.
Results
Abundance
Several hares foraged simultaneously
Typically, one or two hares were recorded on different transects during the same minute of observation time. However, we observed at least seven different hares active simultaneously during December (experiment 1; 10 transects,
Fig. S2), as well as three different hares in January (experiment 2; 4 transects), and four in March (experiment 3; 4 transects,
Fig. S3). Overall, our cameras recorded more than 8500 images. It is thus clear that the patterns we observed and the strategies we analyzed emerge from the population of foraging hares and not from a single individual.
Expectations 1 and 2: Hares should prefer alder over open habitat particularly at the coarse-grained scale—rejected
There was no significant difference in the number of images recorded between habitats in the December experiment (paired t = −1.56, p = 0.153, N = 10). Although sample sizes were small, the same trend persisted in January (paired t = −0.89, p = 0.441, N = 4) and March (paired t = −0.39, p = 0.732, N = 3). We thus reject the expectation that active hares prefer alder habitat.
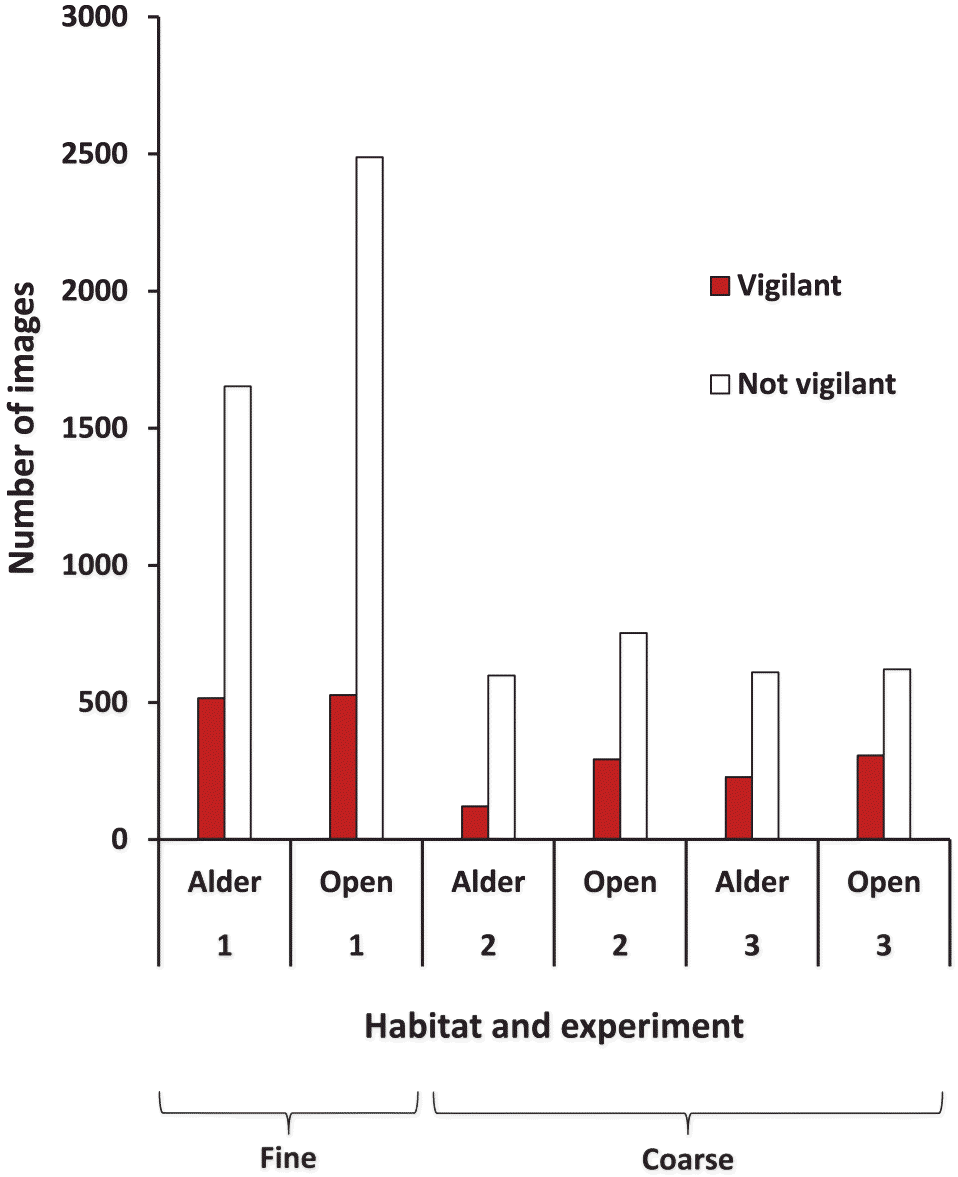
Expectations 3 and 4: Hares should be less vigilant in alder habitat and at sites with supplemental cover—rejected
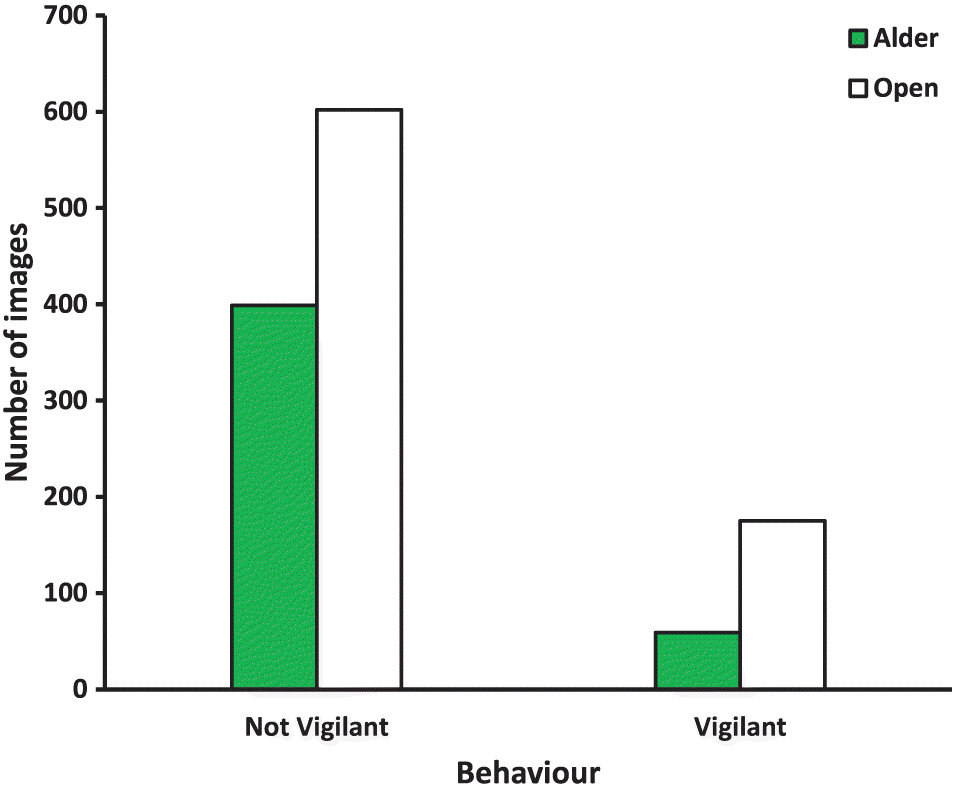
Unexpectedly, a greater proportion of images exhibited vigilance in the alder habitat than in the open (
N = 40 sets of images, saturated model with fixed effects of habitat, treatment, and day;
F1,24 = 5.6,
P = 0.026) during the fine-grained December experiment (
Fig. 3). There was no effect of treatment (tepee versus no tepee), day, or their interactions with habitat (
p ≥ 0.13 for all comparisons) in these analyses.
We also found no evidence that hares were less vigilant under tepees than in the open during March (paired t = −1.18, p = 0.36, N = 3). In both cases, however, there were significantly more images recorded under tepees than in the open (December paired t = −6.61, p < 0.001, N = 10; March paired t = 10.88, p = 0.008, N = 3; more hares under tepees on all transects).
Expectation 5: Hares should be more vigilant in open areas at the coarse-grained scale—tentatively rejected
There was also no clear trend toward higher vigilance in one habitat over the other in either the January or March coarse-grained experiments (January paired t = −1.54, p = 0.222, N = 4; March paired t = −3.36, p = 0.078, N = 3). These results are limited by a small number of transects and must be interpreted cautiously, but there is certainly no compelling reason to accept the expectation that vigilance in risky habitat is necessarily greater in coarse-grained patches than it is in fine-grained patches.
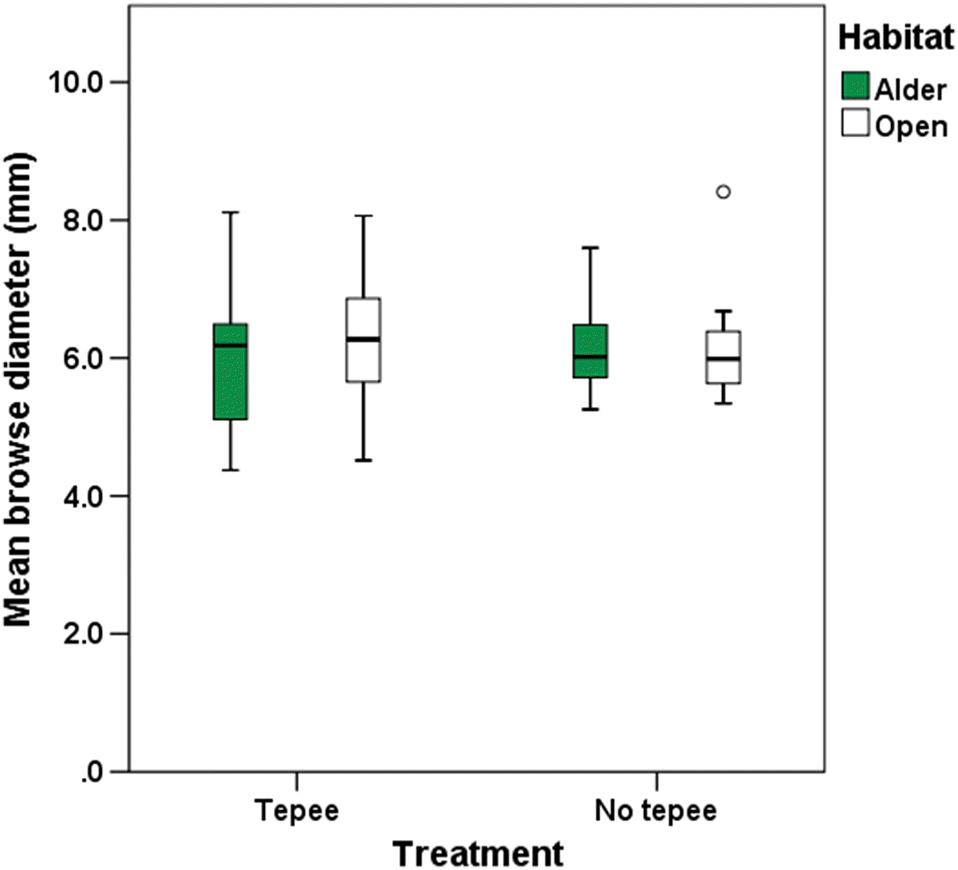
Expectation 6: Hares should forage more tenaciously (lower GUD) when sites are provisioned with supplemental cover—rejected
There were no significant differences or trends in hare GUDs between open and alder habitats or between tepee and no tepee treatments in the December fine-grained experiment (only the intercept (GUD > 0) was statistically significant,
F1,15.3 = 13.3;
p = 0.002;
N = 40, degrees of freedom via Satterthwaite’s approximation,
Fig. 4). These results were confirmed when we tabulated the ratios of the mean paired GUDs by transect, day, and treatment. The number of observations was equal for each of the four comparisons (five each of alder GUD/open GUD > 1 and alder GUD/open GUD < 1 for both tepee and no tepee treatments;
N = 20).
Expectation 7: Vigilance in open areas should increase with distance from alder—tentatively confirmed
Approximately 31% of the images of hares observed between 4 and 10 m displayed vigilance compared with only 21% at 2 m (January experiment, paired t = −3.05, p = 0.055, N = 4).
Expectation 8: GUDs in open areas should increase with distance from alder—confirmed
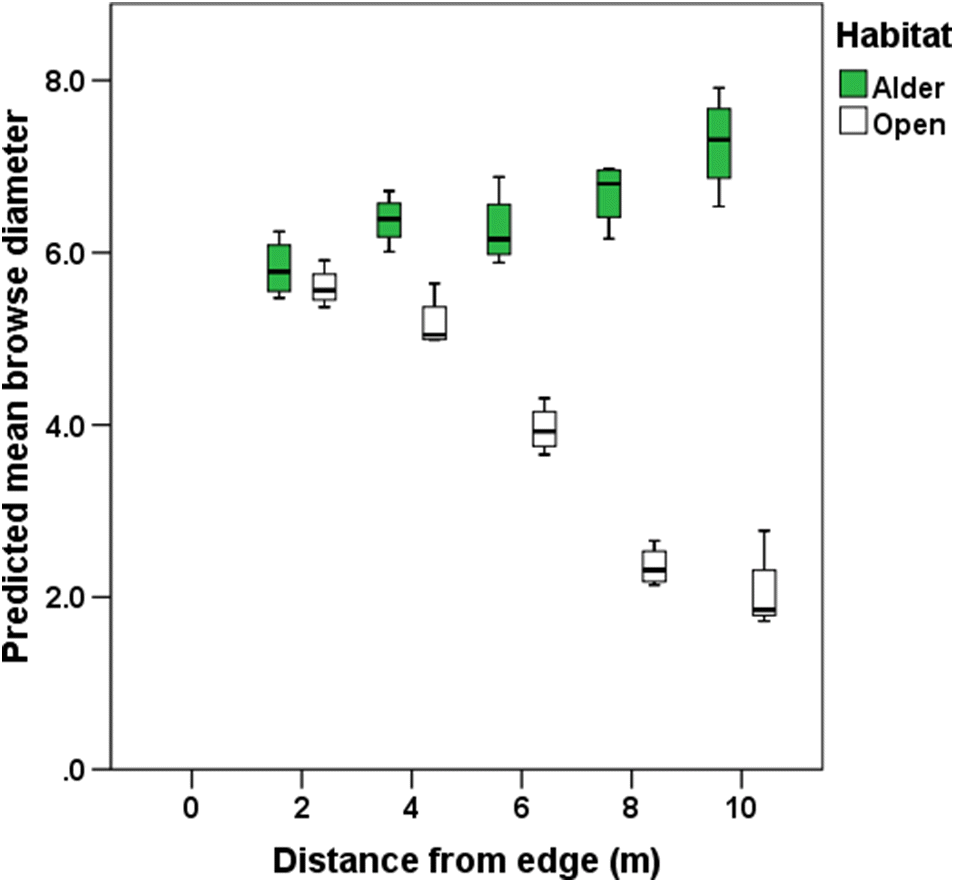
Browse diameters were smaller (GUD higher) in the open than in alder in the January coarse-grained experiment, and they declined with distance. But the decline was asymmetric between the two habitats (habitat × distance interaction,
Table 1,
Fig. 5). Browse diameters varied with vigilance but this effect also depended on habitat (habitat × vigilance interaction,
Table 1). Small browse diameters (larger GUDs) in the open were linked to relatively more vigilant images in that habitat (habitat × vigilance interaction,
Fig. 6).
There was also a slight tendency for GUDs to be higher at greater distances from cover in the March coarse-grained experiment (
Table 2). This effect was not related to supplemental cover (
Table 2) or to vigilance (results for expectations 3–5). There was, however, clear evidence at this scale that GUDs were lower in alder than in the open habitat (
x21 = 16;
P < 0.001;
N = 16,
Table 2).
There was no evidence of bias associated with moon phase or energetic state
There was no difference in GUD (mean browse diameter) for comparable open boughs between the December full moon and January half-moon experiments (
F1,26 = 1.77;
P = 0.195). Changes in moon phase could alter the energetic state of foraging hares if hares tended to avoid foraging during one phase or another. The marginal value of fitness in terms of energy is higher for animals in a low energetic state than it is for those in a high energetic state (
Houston and McNamara 1999). Any difference in mean state will thereby be reflected in the quitting-harvest rate (GUD;
eq. 1). The similarity in GUD between habitats in December (experiment 1) thus suggests that our results were not caused by either moon phase or the foragers’ energetic state.
Discussion
The most striking feature of our experiments is that the hares rejected six of eight possible mechanisms to manage predation risk. Each rejection can be accounted for if hares trade off the benefits of hiding spots and escape routes afforded by alder against the cost of less effective vigilance associated with dense and tangled branches that diminish sight lines (e.g.,
Banks et al. 1999;
Embar et al. 2011;
Iribarren and Kotler 2012a,
2012b). Accordingly, hares were more vigilant in the alder than in the open. In the open, predators should, for a given level of vigilance, be more easily detected. So as long as hares can quickly dart into the safety of alder, less vigilance in the open may not translate into increased risk.
Compensation of foraging risk by the sight line versus escape habitat trade-off will fail if foraging patches exceed the distance required for safe retreat into dense cover. Hares should respond to the reduced safety through higher levels of vigilance and less tenacious foraging. Our experiments, for both vigilance and GUDs (e.g.,
Fig. 5), verify this prediction and suggest that the threshold distance for complete compensation is as short as 4 m. The result is remarkably consistent with
Hodson et al.’s (2010, p. 613) threshold estimate of ≥4 m by hares foraging in canopy gaps.
Even so, you might wonder why GUDs were not different between control patches in the open versus the supplemental cover treatment. More images were observed under supplemental cover, so the cumulative foraging by hares should have been more intense (lower GUDs). This prediction assumes, however, that hares are equally apprehensive in both scenarios. Apprehension, defined as reduced attention to foraging, can reduce food intake and patch assessment (e.g.,
Hochman and Kotler 2007). Apprehension that distracts attention from foraging (such as through resting, listening, or head-down scanning) can reduce the accuracy of a prey individual’s resource assessment and reduce the value of knowledge gained while foraging (
Dall et al. 2001). Each effect will increase the GUD relative to a safer less apprehensive alternative.
Snowshoe hare escape behaviour (measured by the tortuosity of their escape paths) is relatively stereotyped (
Hodges et al. 2014) and they rely heavily on crypsis, immobility, and concealment to reduce detection by predators (
Zimova et al. 2014). It is thus possible that hares employ a secondary trade-off between crypsis (immobility) and foraging. Supplemental cover may interfere with predators’ ability to see motionless hare body forms masked by white pelage in a snow-covered landscape. Cryptic immobility nevertheless increases apprehension, and thus reduces foraging efficiency for stationary hares under supplemental cover. Hares cannot easily conceal their body shape in the open and crypsis is less effective, so hares forage more rapidly and efficiently. Our experiments suggest that the two effects, immobility that produces apprehensive foraging under cover versus more time-efficient foraging in the open, counter-balance one another (no difference in GUDs). This hypothesis awaits testing with more detailed video data.
Our data, and those by
Morris (2005) showing reduced GUDs with distance from cover, are contrary to
Hodson et al. (2010) who did not find an effect of distance on browse diameters in forest gaps.
Hodson et al. (2010) proposed that the absence of a distance effect may have been associated with flat harvest rates on jack pine boughs or by evidence that hares, at least on some occasions, carry harvested boughs to safer habitat. Although we cannot irrevocably rule out these possibilities, it does not appear that they can account for our data. Flat harvest curves are unlikely because we (and
Morris 2005) found distance-dependent differences in GUDs. Consumption of boughs primarily in safe habitat is similarly unlikely because residual pine needles and tracks documented that most boughs were consumed in close proximity to the foraging patch.
Our data suggest that risk management by snowshoe hares is much more sophisticated than the intuition and evidence (
Keith et al. 1984;
Smith et al. 1988;
Hik 1995;
Rohner and Krebs 1996) that hares perceive greater risk in open than in covered habitat. Risk depends on scale (and particularly distance from safety) and can be ameliorated by a multiplicity of compensating strategies. Hares compensate for distance-dependent risk in open habitat with increasing vigilance. Vigilance is only partially effective at reducing scale-dependent risk so hares also forage more apprehensively for less reward (higher GUD).
Our results also add insight to
Hodges and Sinclair’s (2005) conclusion that snowshoe hares in food-enriched sites biased their browsing toward dense cover.
Hodges and Sinclair (2005) compared travel distances, foraging time (fecal density), habitat use, and natural browse among predator exclosure (
N = 1), exclosure plus food (
N = 1), food supplemented (
N = 2), and control (
N = 4) sites. They did not acquire simultaneous estimates of anti-risk behaviours such as vigilance. Our study and those of
Morris (2005) and
Hodson et al. (2010) reveal clear patterns of risk-sensitive behaviours that can assist in averting predation. But it is not common-sense cause and effect whereby safe foraging induces deleterious effects on nutrition, physiology, reproduction, and survival (e.g.,
Hodges and Sinclair 2005, p. 280). Rather, strategies of risk management emerge through scale-dependent foraging options. When the scale of risk is fine-grained, hares reveal that trade-offs between the effectiveness of vigilance (sight lines) and escape habitat compensate for differences in predation risk. Each foraging patch yields similar profit. But as the scale shifts towards coarse grain, increased vigilance with distance from safety fails to fully compensate predation risk, so prey also reduce their foraging effort. A study concentrating on a subset of strategies, or one scale of heterogeneity, would fail to assess the multiple spatial and habitat-contingent strategies that prey can use to defuse the risk of predation. More importantly, it would run the risk of misinterpreting cause and effect and thus misinform the connections linking behaviour with its population dynamic and community consequences.