Risk varied through space and time
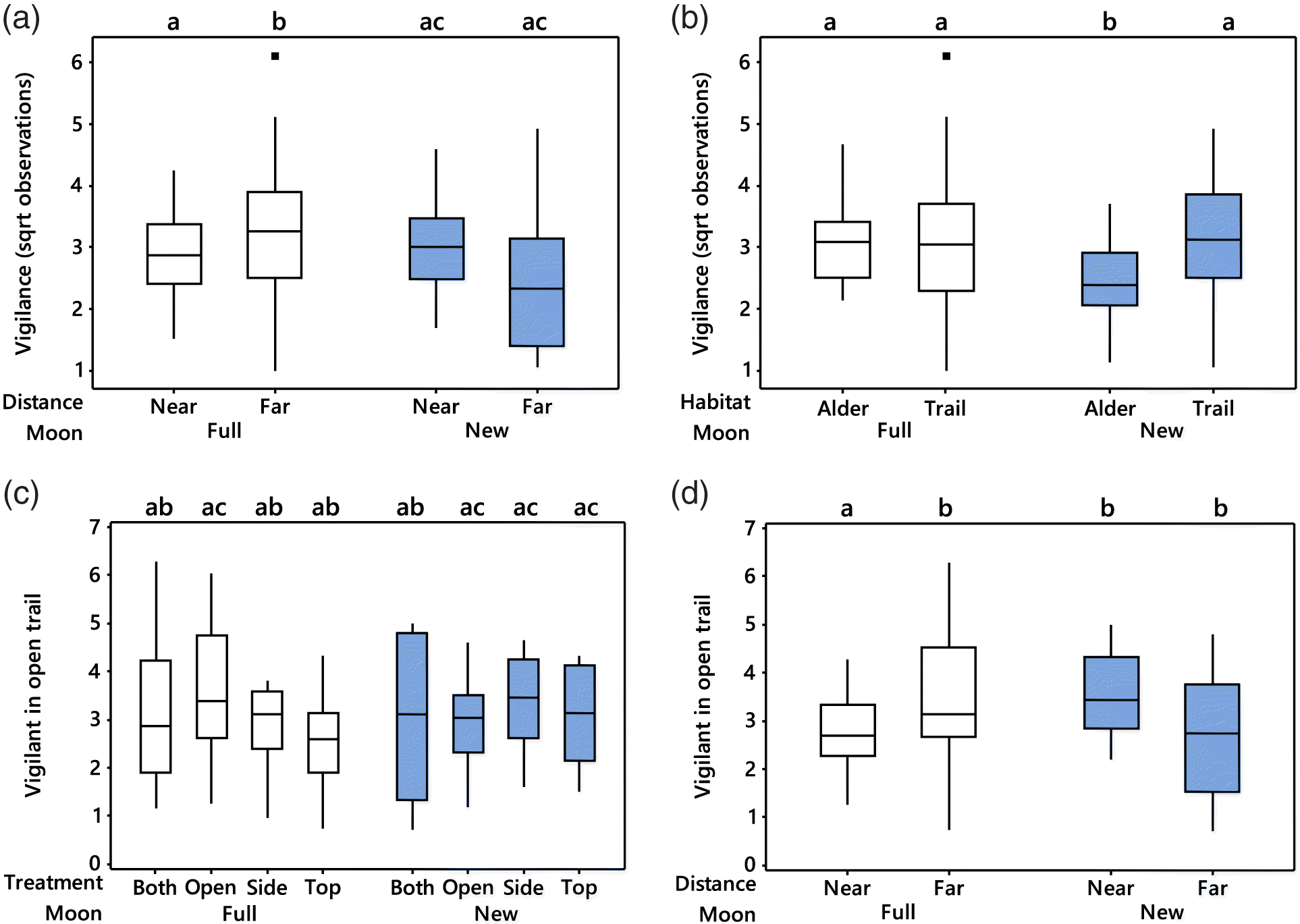
Risk management by vigilance and time allocation, as illustrated by snowshoe hares, is not nearly as complex as it could be. The marginal effects of habitat and distance on vigilance were only apparent in the context of the full versus new moon. Meanwhile, moon phase had no overall detectable direct or indirect effect on GUDs and the main effects of habitat and distance depended on their interaction. These results are a rather stark contrast to the significant differences in vigilance between habitats reported in an earlier study of snowshoe hares foraging pine boughs in open versus alder habitats in the same area (
Morris and Vijayan 2018). Hares in that study were most vigilant in dense alder habitat, opposite to my results demonstrating least vigilance there, but only during the new moon; strategies of risk management thus change through time.
Morris and Vijayan (2018) attributed high vigilance in dense habitat as a trade-off between the ability to elude or escape from predators through the maze of stems and branches afforded by alder and reduced sight lines in the alder that limited visibility required to detect predators. My treatments were designed to test this hypothesis. I reasoned that hares would be most vigilant in the open when their sight lines were restricted by my palisades of alder branches. I included a control and two additional treatments (top and both) to the side treatment in an attempt to clarify how various types of cover alter vigilance and time allocation. Although treatment was included in the two analyses restricted to tests in the open-trail habitat, its only significant effect was on vigilance through a complex interaction with moon phase. If sight lines modulate vigilance, then why was vigilance “reversed” in 2018 and without clear effects of my treatments?
The explanation appears to rest with differences in risk between 2010 and 2018. The clear reduction in predator activity between years suggests that hares were more vigilant and trepidatious toward predators in 2010 than in 2018. Comparison of camera images between the two study periods confirms the prediction. Fully 20% (1043 of 5344) of the images from 2010 revealed alert or vigilant behaviour compared with only 5% (1407 of 25692) in 2018. The proportion of photographs with two or more hares (3% in 2010 vs. 1% in 2018), as well as that of chases (36 in 2010 vs. 14 in 2018), was also strikingly higher in 2010. It is thus reasonable to attribute the higher vigilance observed in 2010 to a higher risk of predation. That risk was exacerbated by heightened competition that reduced the hares’ foraging efficiency.
Hares adjust their vigilance and foraging to match risk
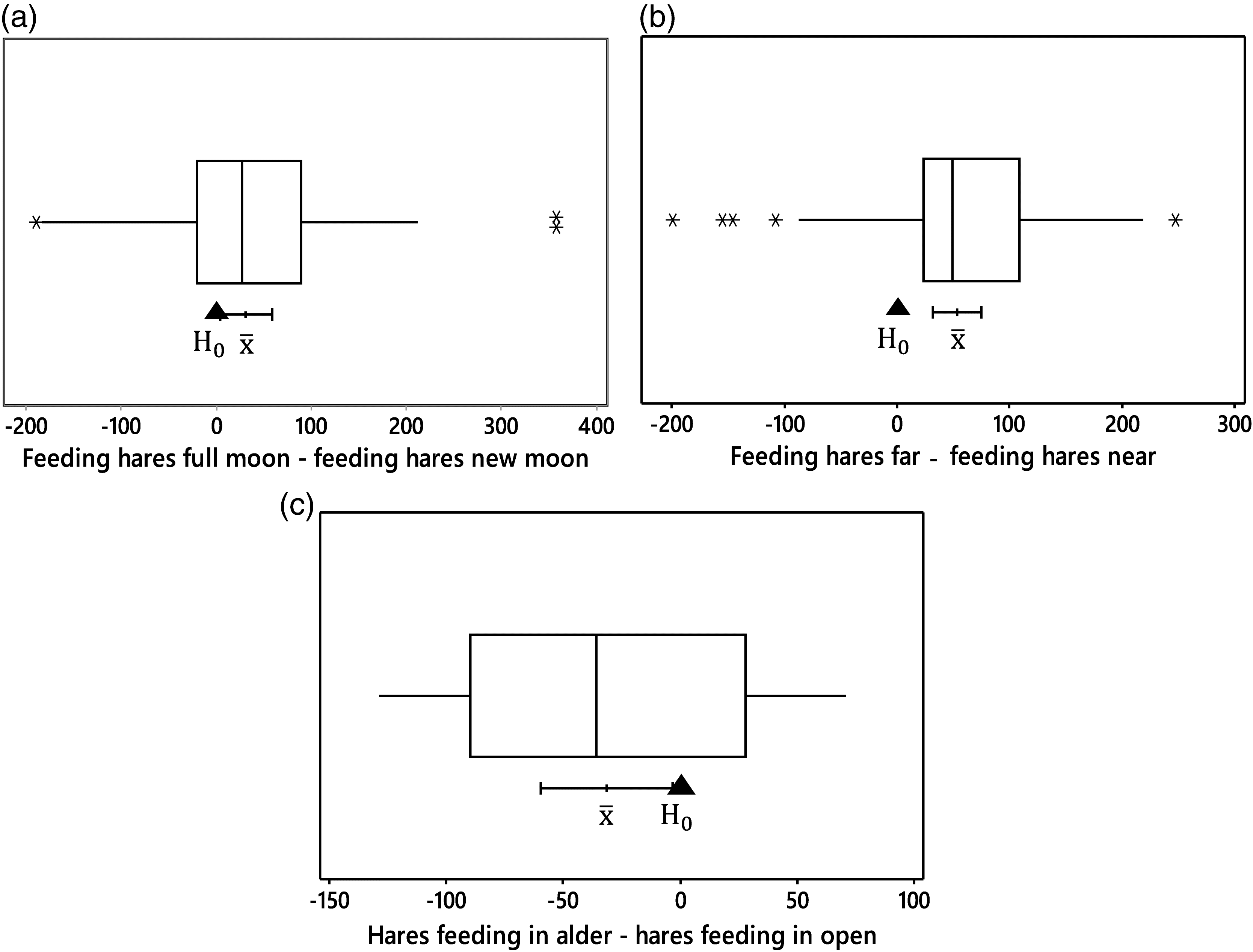
Relationships between risk and its management through trade-offs between vigilance and time allocation by snowshoe hares in 2010 might appear inconsistent with theory. Hares were significantly more vigilant in the alder than in the open habitat. Increased vigilance should normally reduce the efficiency of resource harvest (increase GUD). Mysteriously, there was no difference in GUDs between habitats. The mystery is resolved when one considers that risk of mortality in a habitat must also include what happens after the prey detects a predator. Alder is escape habitat for hares. They evade predators by zig-zagging through its dense tangle of branches. Improved sight lines in the open allow hares to more easily detect approaching predators. But the net effectiveness of their vigilance is reduced because the probability of capture during flight is higher there than in the alder. Hares should be more prone to fleeing and forage more apprehensively, and thus less efficiently, in the open habitat. My data support the hypothesis. The proportion of images with hares foraging in alder was less than in the open (
Fig. 3c).
China et al. (2008) proposed a novel safety in numbers hypothesis (e.g.,
Treisman 1975,
Bednekoff and Lima 1998) for desert gerbils that might also help to explain hare foraging. The gerbils prefer semi-stabilized dunes where winds redistribute food daily (
Kotler et al. 1993). As population sizes increase, competition associated with high density in the rich semi-stabilized habitat causes individuals to shift habitat use towards poorer stabilized dunes. The resulting increase in density there dilutes predation risk for each individual. Reduced risk lowers the value of vigilance, yields more opportunity for foraging and other activities, and promotes additional immigration. Simultaneously, this so-called “risk pump” necessarily drains safety from the semi-stabilized dunes, promoting even more immigration. The system finally attains equilibrium at high density when the GUDs converge (but not vigilance).
Might winter-foraging snowshoe hares pump safety from one habitat to another? Perhaps. My observations of similar GUDs, but divergent vigilance between habitats, is consistent with the risk-pump hypothesis. Hares use vigilance to manage risk from both predators and competing hares. So in order for a risk pump to operate, the reduction in predation risk must outweigh all additional density-dependent costs associated with competition for shared resources.
If we truly understand the trade-offs between vigilance and time allocation, then we must be able to explain patterns of vigilance during the different moon phases in 2018. Two hypotheses are consistent with those patterns.
First, bright moonlight should improve the effectiveness of vigilance as prey scan their surroundings for threats. Hares in the open habitat confirmed this expectation during the full moon by reducing the number of vigilant scans, especially so at near stations where they could quickly dart into the safety of alder (
Fig. 1d). Even so, net risk typically intensifies for nocturnally foraging prey as the moon waxes towards full (
Gilbert and Boutin 1991;
Kotler et al. 2010;
Gigliotti and Diefenbach 2018), so hares were more vigilant at stations where traversing the open habitat took them far from safety (
Fig. 1a) and made them more visible to predators. Second, the hares’ energetic state was almost certainly lower during the cold nights of the full moon relative to those during the new moon. If hares require more energy during cold temperatures, then both vigilance and QHRs should be reduced (
eq. (1)). Neither hypothesis is exclusive of the other, both are likely, and both predict my observation that there were more images of hares foraging during the full than during the new moon (
Fig. 3a).
My data allow one final test. There was no simple difference in vigilance between near and far stations, but GUDs were lower at near stations than at far ones. Hares at near stations should have a lower QHR revealed by fewer images of foraging hares. Once again, data agree with the prediction. There were fewer images of foraging hares at near than at far stations (
Fig. 3b).
Critics might wonder whether patterns of vigilance and foraging were related to concomitant changes in predator and hare density. The experimental design and field observations suggest otherwise. Significant population changes were unlikely given the short 18 d duration of the study, and the number of mammalian predators was effectively zero during the entire experiment.
It thus appears that hares tune their foraging to match the effectiveness of vigilance at reducing risk in different contexts and conditions.
Gigliotti and Diefenbach (2018) reached a similar conclusion: flexible behaviour allows hares to match risk avoidance with local conditions. Vigilance alone cannot ameliorate risk that effects changes in apprehension revealed by GUDs (
Dall et al. 2001;
Kotler et al. 2010). Interactions of foraging and vigilance with habitat, moon phase, and distance from escape habitat highlight a sophisticated risk-management strategy contingent on changes in risk and opportunity. That strategy is nevertheless completely consistent with
Brown’s (1999) theory of optimal trade-offs between vigilance and time allocation.
The agreement between theory and observation in snowshoe hares highlights the importance of adaptive risk management in natural populations. Hares merge their demand for energy with patterns of vigilance and time allocation in a way that maximizes overwinter survival. Their foraging strategy must necessarily impinge on subsequent population dynamics (
Hik 1995) and their numerical interactions with other species. Hare foraging behavior thus adds to a growing body of knowledge gained from clever experiments that documents risk management as a major structuring force in the spatial dynamics and coexistence of species (e.g.,
Kotler et al. 2010;
Camp et al. 2017). Any study of prey foraging that fails to include an assessment of the trade-offs is likely to misrepresent risk management and its important role in the dynamics of populations and communities.