Introduction
The fungi
Fusarium oxysporum f. sp.
lini and
Septoria linicola are causes of fusarium wilt and pasmo, which are two commercially significant diseases of flax (
Linum usitatissimum).
Alternaria spp., have also been found to be very prevalent in harvested flax material and can cause destructive diseases in various plants (
Mankevičienė et al. 2006). Agricultural measures for crop protection include the use of chemicals, resistant plant varieties, biological control, and modification of agricultural practices (
Ribeiro-Quitans 2019). We propose to test members of two classes of flax endogenous compounds, CLPs and PAs, for their potency against pathogens of flax. This information may help identify future breeding targets or plant protection strategies.
Flax seeds are one of the richest sources of CLPs, which are N-to-C cyclized amino acids (
Burnett et al. 2016). Several nutraceutical benefits have been proposed for CLPs, involving antiplatelet, antimalarial, antioxidant, and immunomodulatory activities (
Sharav et al. 2014;
Tan and Zhou 2006). Furthermore, the high thermostability of CLPs may be beneficial in some industrial applications, for example to withstand postharvest heat treatments applied to control insects and microbes (
Lurie and Pedreschi 2014). Despite the range of health and industrial applications under development, the natural biological function of CLPs in plants is unknown; although, a role for these peptides in plant defense has been proposed (
Arnison et al. 2013). For instance, transcripts encoding the precursors of CLP #5 ([1—8-NαC]-linusorb A2) and CLP #14 ([1—8-NαC]-linusorb A1) increased up to 4.7-fold in abundance upon exposure of flax roots to
F. oxysporum (
Burnett et al. 2016). Furthermore, flax CLPs inhibited spore formation by other pathogenic fungi,
Aspergillus flavus (IC
50 240 μg/mL) and
Paecilomyces varioti bainier (IC
50 340 μg/mL) (
Zun et al. 2017).
On the other hand, PAs are natural plant endogenous compounds, with well-known antifungal properties (
Takahashi and Kakehi 2010;
Wojtasik et al. 2015). Polyamines are linear, aliphatic amines, including spermidine (a triamine) and spermine (a tetraamine) (
Wojtasik et al. 2015). Exogenous application of spermines to plants induced defenses against several pathogens, mainly through the activation of the plant immune system (
Seifi and Shelp 2019). It has been previously shown that there is a great increase in the abundance of PA-related transcripts in flax as a response to
F. oxysporum (
Wojtasik et al. 2015;
Ribeiro-Quitans 2019). Additionally, it has been reported that presence of PAs in solid agar medium restricts
Fusarium growth in a dose-dependent manner; statistically significant reductions in the radius of hyphal growth were observed with a minimum of 10 mM (880 μg/mL) putrescine, 6 mM spermidine (871.5 μg/mL), and 3 mM (607.02 μg/mL) spermine (
Wojtasik et al. 2015). It should also be noted that the exogenous application of polyamines to plants can disrupt normal metabolic and developmental processes (e.g., pollen germination and elongation), leading to toxic effects (
Wolukau et al. 2004;
Setia and Setia 2018;
Sorkheh et al. 2011).
To investigate whether CLPs could inhibit fungal growth in vitro, we obtained a commercially available mixture of six unique CLP and performed a microdilution assay to test the activity of this CLP mixture against three potential plant pathogens (F. oxysporum, S. linicola, and Alternaria sp.). We compared the efficacy of the CLP mixture to carbendazim, a broad-spectrum commercial fungicide, and two PAs, spermidine and spermine. We chose these PAs to represent low molecular weight, endogenous compounds that have previously been shown to inhibit fungal growth.
Material and Methods
Fungal sources and culture
Fusarium oxysporum f.sp. lini isolates 13 and 81 were generously provided on potato dextrose agar (PDA) by Dr. Khalid Rashid (Agriculture and Agri-Food Canada, Morden, MB, Canada) and Dr. Martin Reaney (University of Saskatchewan), respectively. Dr. Reaney also provided Septoria linicola isolate (14SL43) in the same conditions. Alternaria sp. isolate (BH 503) was provided by Dr. Louise Nelson (University of British Columbia). It should be noted that there has been no controlled assessment of the pathogeneticy of isolate BH 503.
Cultures were grown on PDA at room temperature in the dark until growth covered the plates (approximately 14 days). Spores were isolated by flooding the plate with sterile water with 1% Tween 20 and gently rubbing with a sterilized loop. The solution was filtered using a sterile cheesecloth to isolate the spores and remove the mycelia. Spore concentration was quantified using a hemocytometer to the required final concentration of 105 spores in each well of the microdilution assay.
Test compound sources and dilutions
A CLP mixture extracted from
L. usitatissimum was kindly provided as a lyophilized powder by Martin Reaney (Prairie Tide Diversified Inc.; CLMIX, Lot M201304-001). The CLPs were tested as a mixture because this product is commercially available, is representative of the most abundant CLPs in flax, and pure isolates of individual CLPs are prohibitively expensive. The composition of this powder is listed in
Table 1, and the composition-weighted mean molecular mass of the mixture is 1051.6 g/mol. Other test compounds were purchased: carbendazim (Sigma 378674, 97% pure, 191.19 g/mol, CAS 10605-21-7), spermidine (VWR CAAAA19096-06, 99% pure, 145.25 g/mol, CAS 124-20-9), and spermine (VWR CAAAAL19562-06, 97% pure, 202.35 g/mol, CAS 71-44-3).
In vitro inhibitory assays for of the CLP mixture and carbendazim followed the standard broth dilution method (
EUCAST 2019), with a few modifications. Briefly, the CLP mixture and carbendazim were dissolved in dimethyl sulfoxide (DMSO) (Sigma, St. Louis, MO) at 200× the desired final concentration. A two-fold dilution series was prepared in DMSO, with 10 different concentrations. Subsequently, another dilution was performed by taking 100 μL of each tube with 200× concentration of the substance to be tested and transferring this to 9.9 mL of malt extract 2% medium (VWR, 97063-426) (1:100 dilution). This reduced the concentration of the solvent in the culture tubes to 1% and the concentration of the tested compounds to 2×. For experiments on the effect of PAs, the highest concentration was prepared at 5× the final concentration in sterile water. A two-fold dilution series was prepared from that in water, with 10 different concentrations.
Microdilution assays were performed in sterile 96-well polystyrene plates (Greiner Bio-One, Monroe, NC). The CLP mixture concentration ranged from 0 to 512 μg/mL and the concentration of carbendazim ranged from 0 to 50 μg/mL. For the CLP mixture and carbendazim assays, each plate was prepared by adding 100 μL of the 2× final concentration of the compound in 2% malt extract into the wells of the columns 1 to 10. For example, for the CLP mixture, we dispensed to each well in column 1 the medium containing 512 μg/mL of CLP mixture, 256 μg/mL to column 2, 128 μg/mL to column 3, and so forth. Wells from the columns 11 and 12 had only the media, without the tested compounds.
The concentrations for the PA assay were chosen based on a previously published study (
Wojtasik et al. 2015). The highest concentration for the 2× dilution was 10 mM for each PA, which corresponds to 1452 μg/mL of spermidine and 2023 μg/mL of spermine. Each plate was prepared by adding 20 μL of the 5× final concentration of the compound in water into the wells of the columns 1 to 10. Wells from columns 11 and 12 had only 20 μL of water, without the compounds.
Fungal growth inhibition assay
For the CLP mixture and carbendazim, we added 100 μL of the spores suspension to each well of the plate (except for the column 12, where it was the negative control) to achieve the final concentration of 1 × 10
5/mL, as recommended by
EUCAST (2019); 100 μL of the sterile DI water used to prepare the spore dilution was added to column 12. The
in vitro inhibitory assays of PAs were performed according to
Zeitler et al. (2013). An 80 μL aliquot of fungal spores at 10
5 spores/mL resuspended in 2% malt extract media was added per well resulting in final fungi concentration of 1 × 10
5/mL spores and a final compound concentration of 0–10 mM of each polyamine, which corresponds to: spermidine (0–1452 μg/mL) and spermine (0–2023 μg/mL). We added 100 μL of the water used to prepare the spore dilution to column 12.
An initial absorbance reading at λ = 530 nm was recorded for all the plates using the Varioskan LUX Multimode Microplate Reader. After incubation for 2 days at room temperature in the dark (and 3 days for S. linicola), fungal growth was determined by measuring optical density (OD530nm) with the Varioskan LUX Multimode Microplate Reader (Thermo Fisher Scientific, Waltham, MA) and by visual inspection.
The median effective concentration (EC
50) was calculated as the concentration at the midpoint between the baseline growth rate and the dose point at which maximum inhibition occurred. The EC
50 values were calculated using the drc package in R (
Ritz et al. 2015). We performed at least two biological independent experiments for each assay, with eight technical replicates per experiment, for a total of 16 technical replicates across all experiments.
Discussion
The natural biological roles of orbitides (i.e., CLPs) are not well established. Because of the hydrophobic composition of CLPs, it has been suggested that they may have a membrane-active role, possibly resembling antimicrobial peptides (AMPs) (
Arnison et al. 2013;
Arouri et al. 2011). However, CLPs differ from AMPs, because the CLPs identified so far are not cationic. Therefore, we were motivated to test whether a mixture of CLPs isolated from
L. usitatissimum could inhibit growth of three fungi commonly associated with plants:
F. oxysporum,
S. linicola, and
Alternaria sp. Of these,
F. oxysporum and
S. linicola are significant pathogens of flax, but the pathogenicity of this local
Alternaria sp. isolate has not been established. For comparison, we also assayed a commercial fungicide (carbendazim) and two PAs that have previously been shown to inhibit growth of some of these fungi in other types of assays (
Amini and Sidovich 2010;
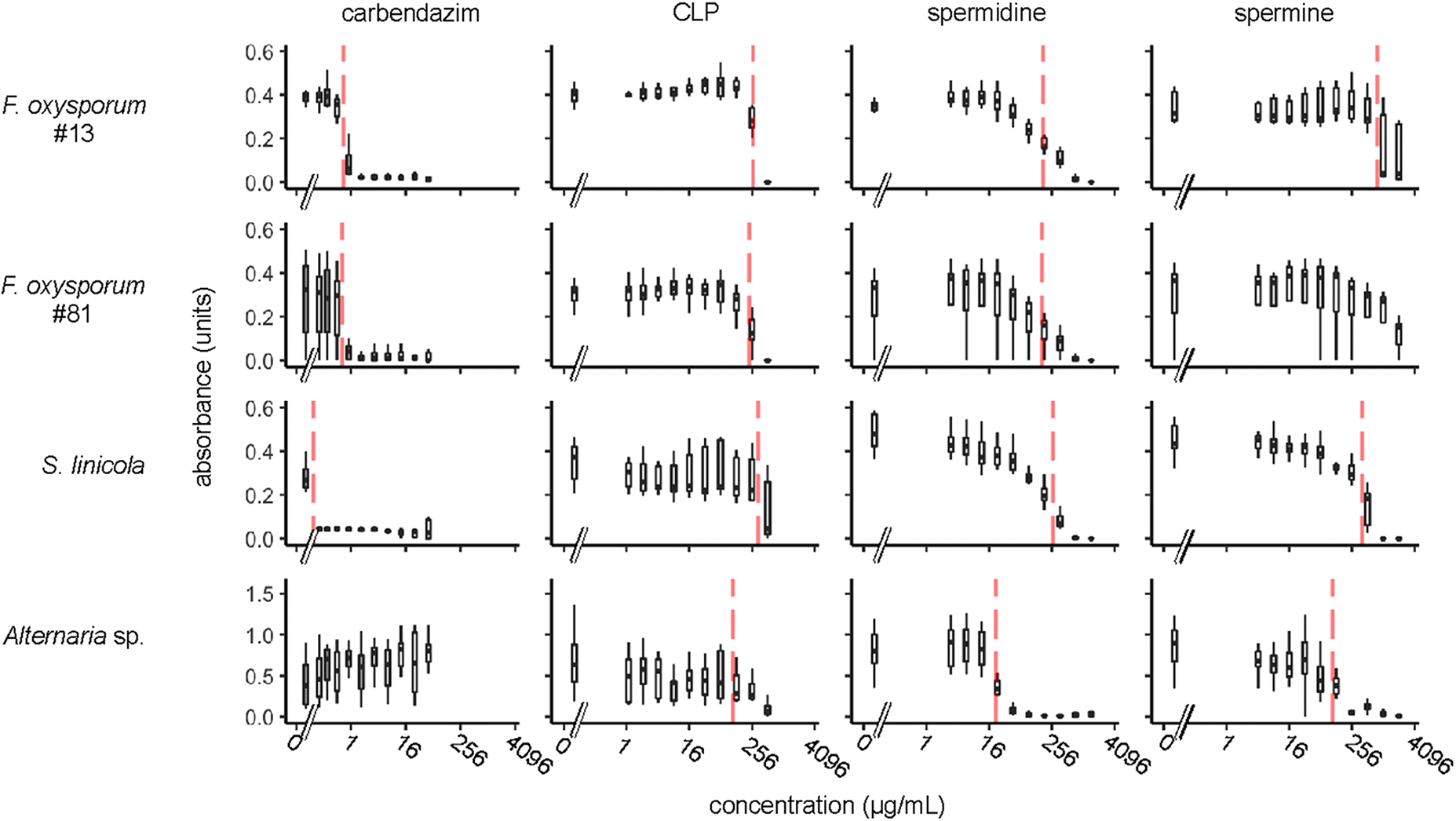
Wojtasik et al. 2015). We found that the median effective concentration (EC
50) for the CLP mixture in our growth inhibition assays ranged from 111 to 340 μg/mL (
Table 2). These results are consistent with the EC
50 ranges reported by
Zun et al. (2017) for CLPs with two other fungi. In our study, for each fungus assayed, EC
50 values were slightly higher for the CLP mixture than for spermidine (21–272 μg/mL) and lower or similar for the CLP mixture than spermine (109–778 μg/mL). In contrast, EC
50 values for carbendazim for
F. oxysporum and
S. linicola (<0.58 μg/mL) were much lower than the CLP mixture or PAs. Thus, we conclude that both the CLP mixture and PAs have measurable fungicidal activity, but their potency against
F. oxysporum and
S. linicola is weak when compared to a commercial fungicide.
Alternaria spp., are known to be resistant to benzimidazole fungicides such as carbendazim, and this was consistent with our observations (
Eckert and Ogawa 1985). However, spermidine was more effective against
Alternaria sp. (EC
50 21 μg/mL) than either of the other fungi we tested. These results raise an interesting question: Is the efficacy of spermidine on
Alternaria sp. related the necrotrophic lifestyle of the fungus? It has been suggested that there are differences on PA biosynthesis induced by either biotrophic or necrotrophic pathogens in plants (
Pal and Janda 2017); however, the role of PA metabolism in the interaction of plants with pathogens with different lifestyles is still not clear. Further work is required to establish the role of free spermidines on the growth inhibition of necrotrophic pathogens, such as
Alternaria spp. It must further be emphasized that although the isolate used in these experiments were obtained from diseased plant tissue, the pathogenicity of this isolate has not been established. Therefore, there are potentially many explanations for the observed sensitivity of this isolate to spermidine. Furthermore, any potential applications of exogenous PAs as fungicides must take into consideration both the established and potentially still unknown toxic effects of PA application on plants (
Wolukau et al. 2004;
Setia and Setia 2018;
Sorkheh et al. 2011).
Although for both
F. oxysporum and
S. linicola, all of the natural compounds we tested were orders of magnitude weaker as fungicides than carbendazim, the effective ranges we observed for some of these natural compounds are near to what might be present
in planta, under some conditions. For example, the concentration of free spermidine in flax seedlings exposed to
F. oxysporum is 10 μg/g of fresh weight, with the inclusion of conjugated and bound forms of spermidine this increases to 65 μg/g of fresh weight (
Wojtasik et al. 2015). The EC
50 we observed for spermidine ranged from 21 to 272 μg/mL, and suppression of fungal growth was evident far below the median (
Fig. 1). Therefore, we cannot yet exclude the possibility that spermidine has some antifungal activity in the phyllosphere, rhizosphere, or within the plant itself. Furthermore, our results show that free spermidine can inhibit fungal growth, even in the absence of conjugation to other plant-produced molecules or induction of plant signaling cascades. Thus spermidine may have a direct role in modulating fungal growth, in addition to its many other roles in defense and other processes, both within fungi and within plants (
Jiménez-Bremont et al. 2014;
Pal and Janda 2017). On the other hand, spermine was less potent than spermidine in our assays. Moreover, endogenous spermine is less abundant in flax seedlings than spermidine. Thus, the reported ability of spermine to promote resistance to disease may be due to the modulation of the plant immune system rather than direct fungicidal activity (
Seifi and Shelp 2019;
Takahashi 2016).
Flax is one of the richest natural sources of CLPs, and the concentration of CLPs in flax seeds can be as high as 300 μg/g (
Arnison et al. 2013;
Gui et al. 2012). Concentrations of CLPs are likely much lower in roots, but it is possible that CLP abundance could be increased in response to a pathogen
in vivo. It also remains possible that CLPs could act more effectively as fungicides
in planta, as part of multicomponent defenses. This is consistent with the ideas of
Fisher et al. (2018), who suggested that PawL-derived Peptides, orbitides from the subfamily Asteroideae, might have antibacterial effects
in vivo working as multicomponent antimicrobials (
Fisher et al. 2018;
Garneau et al. 2002). Further studies on the current topic are therefore recommended, to elucidate synergistic effects of CLPs
in vivo.
Conclusion
F. oxysporum,
S. linicola, and
Alternaria sp. were each affected by at least one of the compounds studied (polyamines, carbendazim, and a mixture of CLPs), where spermidines were the most effective natural occurring compound against phytopathogens, especially
Alternaria sp, on a mass/volume basis, and the CLP mixture was most effective on a molar basis. Conversely, carbendazim application did not inhibit the growth of
Alternaria sp., whereas this fungicide greatly affected the growth of
F. oxysporum and
S. linicola in vitro. Although the CLP mixture and spermidine were much less potent than carbendazim, both the CLP mixture and spermidine showed fungicidal activity within a concentration range that might be biologically relevant. Moreover, these results show that a CLP mixture and spermidine can have direct effect on fungal growth, independent of any other contributions from the plant host. The findings reported here shed new light on the participation of CLPs in the protection of plants against pathogens in plants, and as well the inhibitory effects of free spermidines on fungi, especially those with a necrotrophic lifestyle. These results open new opportunities for studies to test the effects of exogenous applications of CLPs and (or) spermidines on fungal infection
in vivo and may suggest targets for future genetic manipulation of flax to obtain new lines resistant to diseases. However, the complexity of the ecosystems in the agricultural field also requires several tests to determine the effects of a putative fungicide on plant resistance and yields, as well the effects on the environment, including the impact on the soil microbiota and beneficial interactions with the existing fungi (
Stachowicz 2001).