Introduction
Information emerging from research in archaeology and historical ecology, as well as work with traditional knowledge holders on the Pacific Coast of North America, indicate that Indigenous peoples have had an active role in managing terrestrial, coastal, and aquatic ecosystems over millennia (
Deur 2005;
Moss 2011;
Mathews and Turner 2017). Such research highlights a diverse and well-established set of stewardship practices informed by Indigenous knowledge, including size-selective fishing technology, plant and shellfish cultivation, rights-based harvesting restrictions, and ceremonial controls that enhanced the productivity and availability of numerous habitats and resources (
Deur 2005;
Haggan et al. 2006;
Turner and Berkes 2006;
Menzies and Butler 2007;
Moss 2011;
Lepofsky et al. 2015). The occurrence and persistence of these Indigenous resource management practices contributed to the resilience of coastal marine ecosystems, which challenge notions of Indigenous peoples as “hunter-gatherers” or “fisher-hunter-gatherers” (
Deur and Turner 2005;
Moss 2011). The ongoing legacies of these sustained and intentional activities are being further examined through community-oriented research projects that draw together a variety of techniques and knowledge sources, including archaeological data, with the aim to restore ecosystem function as well as revitalize relationships between people and marine foods (
Augustine and Dearden 2014;
Hatch et al. 2023).
Shellfish are among the most prolific and ubiquitous evidence of edible foods encountered in archaeological sites throughout the Northwest Coast (
Moss 2013). Several species of clam and mussel commonly occur, with littleneck (
Leukoma staminea), butter (
Saxidomus gigantea), and horse (
Tresus nuttallii) among the most prominent bivalves. These burrowing intertidal animals occur in soft sediments (i.e., mud, sand, gravel, and mixed substrates) and are harvested as a reliable and nutritious source of food extending back to some of the earliest documented settlements in the region that have preserved evidence (e.g.,
Fedje et al. 1996;
Cannon et al. 2008;
McLaren et al. 2011;
Toniello et al. 2019). As documented in ethnographic accounts and oral histories, clams have key cultural significance (
Moss 1993,
2013;
Deur et al. 2015) in addition to their important role in local economies and food systems (
Ellis and Swan 1981;
Deur et al. 2015). This emphasis is consistent with archaeological evidence, where massive quantities of clams and other invertebrate species occur in “shell midden” deposits observed throughout the coast. Landforms composed of abundant shell have also been interpreted as monumental landscape features, where previous harvests are purposefully used as geoengineering material, altering coastal geomorphology, and shoreline configurations (
Blukis Onat 1985;
Grier et al. 2017;
Letham et al. 2020). Archaeological investigations into past shellfish harvesting locations, coupled with traditional knowledge and ecological science, have demonstrated the enduring use of intertidal “clam garden” features. Clam gardens are cleared beaches with rock-wall terraces built at low tide, which elevate the beach and trap sediment thereby increasing clam habitat, growth and productivity, harvesting accessibility, and help ensure food security for coastal communities (
Groesbeck et al. 2014;
Lepofsky et al. 2015;
Jackley et al. 2016;
Lepofsky et al. 2021).
However, despite the recognized importance of clams and other invertebrates, quantitative analyses of recovered shellfish assemblages remain infrequently reported in archaeological research. To date, research on Northwest Coast shellfish has largely involved evaluating taxonomic abundances based on count and weight data obtained from sediment samples with a strong focus on seasonality and harvest intensity (
Ham and Irvine 1975;
Keen 1979;
Cannon et al. 2008). More recent sclerochronological studies have involved high resolution δ
18O analysis, offering both palaeoceanographic proxy data and season-at-harvest estimates (
Daniels 2009;
Burchell et al. 2013). For example,
Leclerc et al. (2023) examined 30 butter clam shells recovered from four archaeological assemblages on the south coast of BC to measure seasonality and palaeotemperature trends spanning the last 1000 years. Additionally, this study assessed harvest intensity by comparing the proportion of senile and mature-stage clams using 662 thin-sectioned shell margins following the methods in
Cannon and Burchell (2009). Such comparative approaches assessing clam growth stages (i.e., juvenile, mature, senile) indicate that shellfish were harvested less intensively in the vicinity of long-term residential sites as opposed to short-term encampments (
Cannon and Burchell 2009). Working to integrate archaeology, ecology, and Indigenous knowledge relating to clam gardens, research teams on Quadra Island (BC) examined butter clam age, size, and growth rates from walled and nonwalled beaches to assess the relative role of clam gardens and environmental conditions in promoting clam growth throughout the Holocene (
Toniello et al. 2019). While these and similar studies have substantial analytical utility, these approaches are often relatively expensive and time-consuming, and due to fragmentation, often lack size information.
Considering existing archaeological approaches for characterizing shellfisheries, size represents a foundational metric for comparing harvests and evaluating harvest pressure, both spatially and temporally (e.g.,
Botkin 1980;
Mannino and Thomas 2002;
Braje et al. 2007;
Erlandson et al. 2008;
Thakar 2012;
Klein and Steele 2013). Yet, zooarchaeological research examining shell size remains rare. To date, only a handful of studies include size data, although they are typically limited by the number of intact valves, as most shellfish remains in archaeological sites are fragmented due to compaction, weathering, and the passage of time. While some archaeologists have developed predictive equations to estimate the sizes of individual clam valves using contemporary specimens (e.g.,
Daniels 2014;
Grone 2020), these studies have been limited to a specific species or study area and have tended to ignore geographic variability and measurement uncertainty. Earlier research (e.g.,
Croes 1992;
Moss 1993) rely on “average” meat weights using modern clams and apply these formulae to estimate the proportional biomass (g) of recovered shellfish remains to measure the contribution of shellfish to human diets. Whereas such approaches help address a key aspect of Indigenous foodways, the lack of detailed size data limits current understandings of the cultural and environmental factors that influence clam harvest profiles (e.g., management protocols and practices, predator-prey relationships, ecological productivity, and relative stability in size-at-harvest profiles).
Here, we show that a regression-based approach utilizing shell fragments provides basic size data that are relevant for understanding historical baselines and past resource management strategies. As shell size is a foundational metric that can enable size-at-age biomass and caloric value estimates, research examining size-at-harvest—irrespective of age—is an important first step in characterizing shellfisheries practices. Moreover, since size is the most basic and easily determined metric for broadly assessing shellfish across sites and is integral to contemporary management (i.e., legal size limit), more attention to shell size is needed.
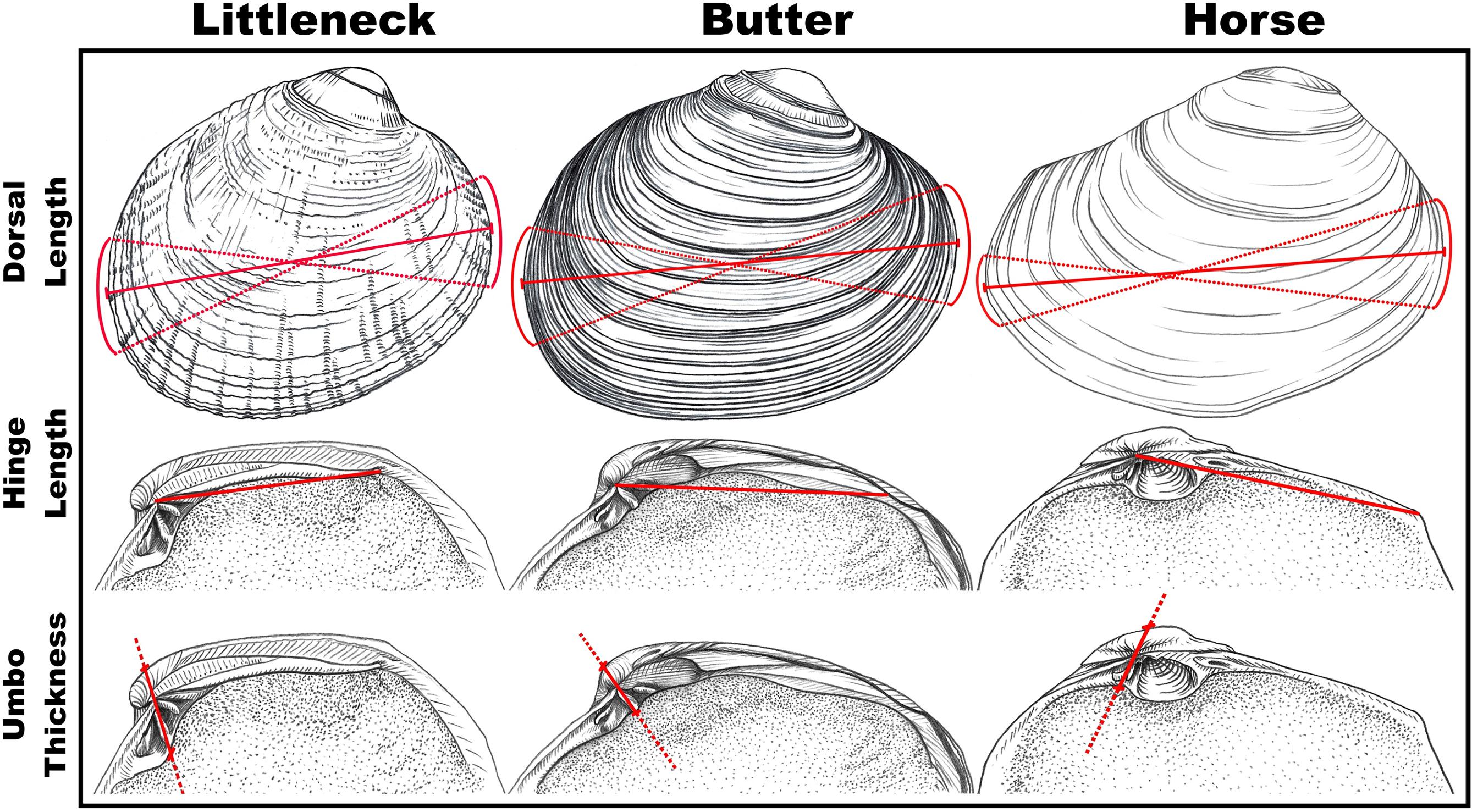
Considering the allometric scaling relationship between the morphological features of an organism and its body size, archaeologists are increasingly applying methods that can infer the body size distributions of prey harvested by people based on partial or fragmentary remains. In coastal archaeology, such scaling relationships have been observed for a range of fish and shellfish taxa, including California mussel (
Mytilus californianus) (
Campbell and Braje 2015;
Singh et al. 2015;
Singh and McKechnie 2015;
Braje et al. 2018). These studies have shown how shell length can be accurately predicted using measurements of morphological landmarks, such as the umbo and hinge, which demonstrate strong linear relationships.
Like other molluscs, clam growth is nonlinear (i.e., growth rates slow once reaching maturity). While the relationship between size and age is nonlinear, there is a general allometric scaling relationship between an individual’s overall size and other morphological features. For each species of clam considered here, maturity is typically achieved around 3 years (
Table 1). Based on these life history characteristics (
Table 1), managers have established size limits for legal harvests (i.e., minimum size limit), which in BC are 35 mm for littleneck and 55 mm for butter clam, while there is no size limit for horse clam although harvesters are limited to six individuals (
Department of Fisheries and Oceans 2023). These size limits are based on contemporary biological data for size at maturity, which incorporate size-at-age and are intended to allow clams the opportunity to spawn at least once before achieving legal size (
Bourne 1987). While size-at-first reproduction is a plastic life history trait in marine invertebrates and can be driven by predation or other size-selective pressures (
Hadfield and Strathmann 1996;
Chase 1999), it is a generalized biological observation which often forms the basis for contemporary conservation and management.
Like all organisms, a host of environmental and biological factors can influence the growth, morphology, and body size of shellfish across ecological settings and evolutionary time scales. For example, temperature, pH, productivity, nutrient runoff, and wave exposure have been shown to influence mussel growth, shell thickness, and size (
Suchanek 1981;
Blanchette et al. 2007;
Pfister et al. 2016;
Black et al. 2017;
Darrow et al. 2017). Another factor influencing size and morphology is the effects of predators, such as sea otters (
Enhydra lutris), which reduce the size structure of bivalve populations (
Kvitek et al. 1992;
Singh et al. 2013). On northern Quadra Island in coastal BC, clam growth rates and sizes have been shown to vary across different time periods over the past 11 500 years, owing to a combination of changing environmental conditions and varying Indigenous stewardship practices (
Toniello et al. 2019). On Vancouver Island specifically, clam growth rates and size are also known to vary geographically in association with environmental conditions (
Foster 2021). Yet, growth rates (i.e., size-at-age) are often difficult to assess in archaeological contexts, and in some cases is challenging to decouple the environmental conditions that influence clam growth (e.g., productivity, temperature, etc.) from cultivation protocols that are known to influence shellfish growth (i.e., clam gardens) (
Groesbeck et al. 2014;
Jackley et al. 2016). However, allometric scaling relationships pertaining to size remain generally constant despite the rates of growth that conspecifics can experience under different conditions. Given the many sources of variation in clam growth and size, the question arises: does this variation influence the morphology of shell valves and their scaling relationship to body size? Furthermore, can these relationships be applied reliably in archaeological settings where fragmentary shellfish remains are common and tend to have low chronological and spatial resolution?
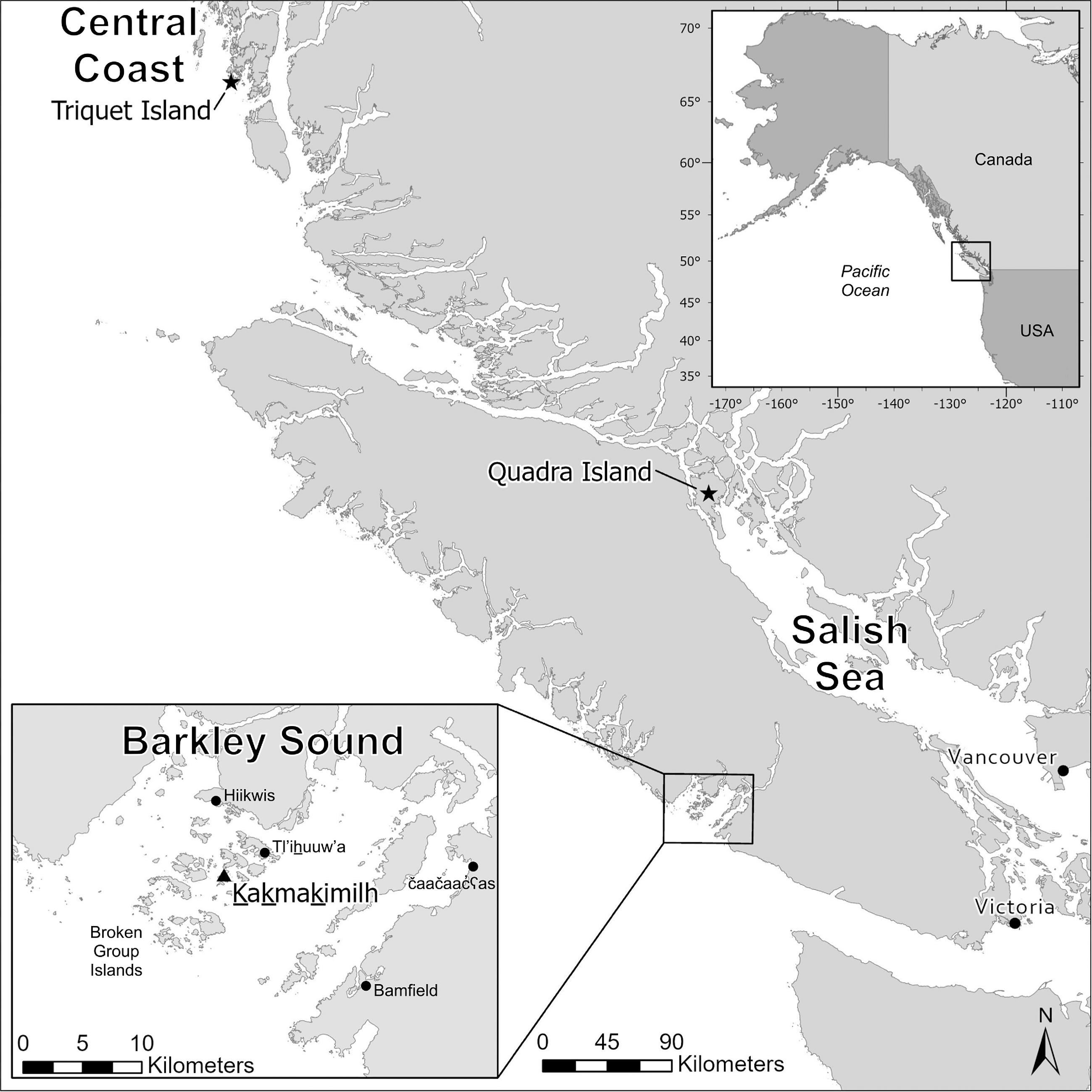
Here, we present a measurement-based method for determining shell length (mm) from the fragmentary remains of littleneck, butter, and horse clams using linear regression formulae. We examine the strength and regional applicability of these formulae and develop measures of uncertainty and reliability for three regions in coastal BC, Canada. We then apply these to demonstrate continuity in archaeological clamshell sizes from a 3000-year-old assemblage in Tseshaht First Nation Territory on western Vancouver Island. This approach can be used to better understand historic shellfisheries’ practices and provide researchers an opportunity to assess magnitudes of change in size-at-harvest over millennia of human-clam relationships on the Northwest Coast. Given the limited information relating to long-term social–ecological interactions in the region, further efforts aimed at generating preindustrial size-at-harvest information for these commercially and culturally valued species can contribute to resilient marine resource management policies and practices today. Here, we aim to offer a simple, broadly applicable and reliable method to estimate shell size in archaeological settings, which represents a fundamental step in characterizing ancient Indigenous shellfisheries’ practices.
Discussion
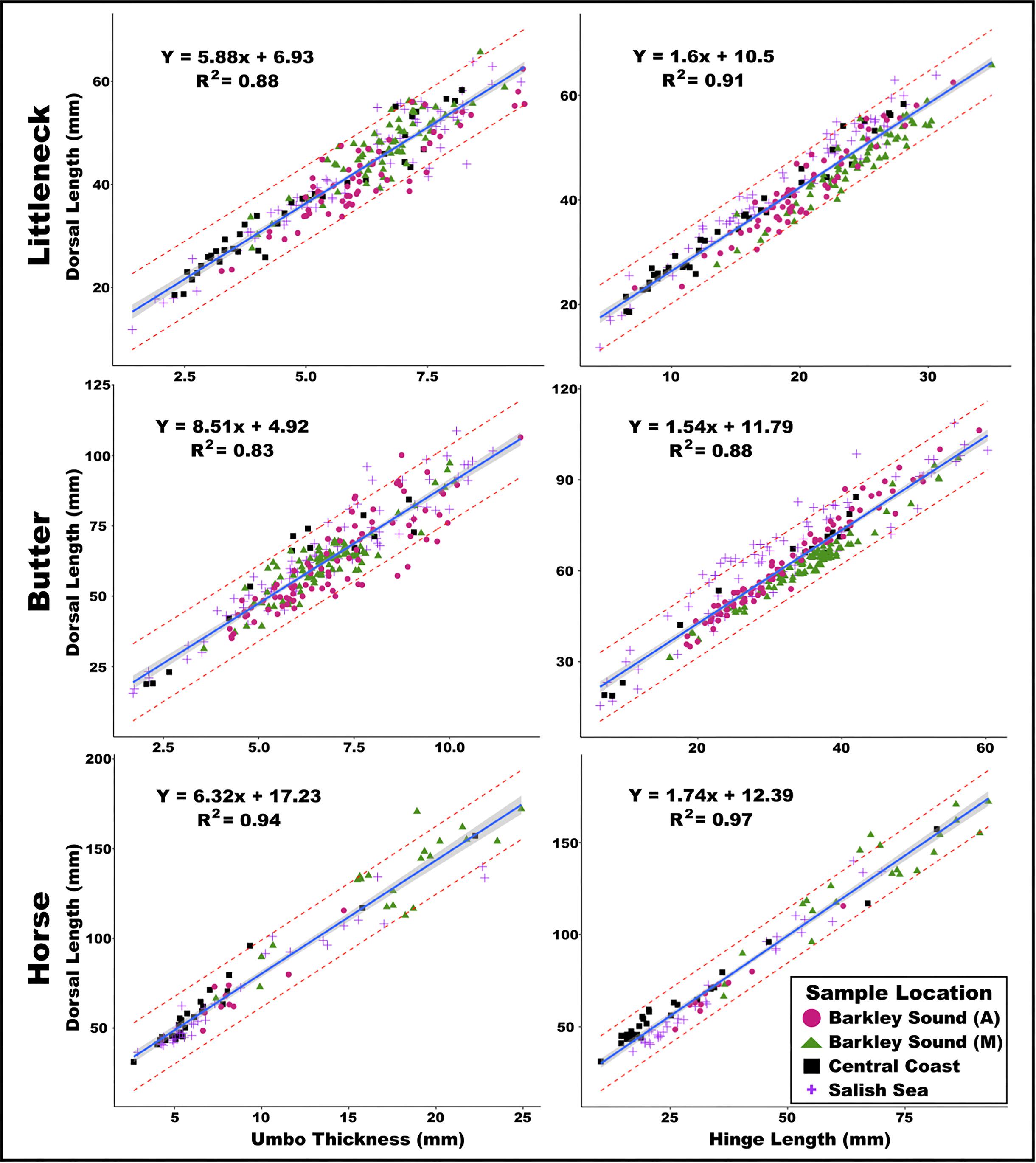
We observe a strong linear relationship between the length of the hinge, the thickness of the umbo, and the dorsal length of littleneck, butter, and horse clams, irrespective of the sampling region or temporal periods examined in this study. For the species considered in this analysis, 83%–97% of the variation in shell length is captured in these linear regression models. These findings indicate that this method provides an effective technique for estimating the dorsal length of clam shells from fragmentary valves, which are overwhelmingly common in archaeological deposits. While differences in ecological processes between regions and time periods can certainly influence the harvested size of clams, the allometric relationship between these variables adequately captures such variation. Furthermore, the strength of these linear regressions highlights the value of using a simple method as a widely applicable first step in understanding broad spatiotemporal trends in harvested clam size. Moreover, as clam shell fragments regularly comprise the majority of coastal shell-midden deposits across the Northwest Coast, this method has the potential to repurpose an underutilized and overlooked source of archaeological information, as well as retrospectively add detail to existing sclerochronological studies where analysis occurred on fragmentary shells retaining the umbo or hinge.
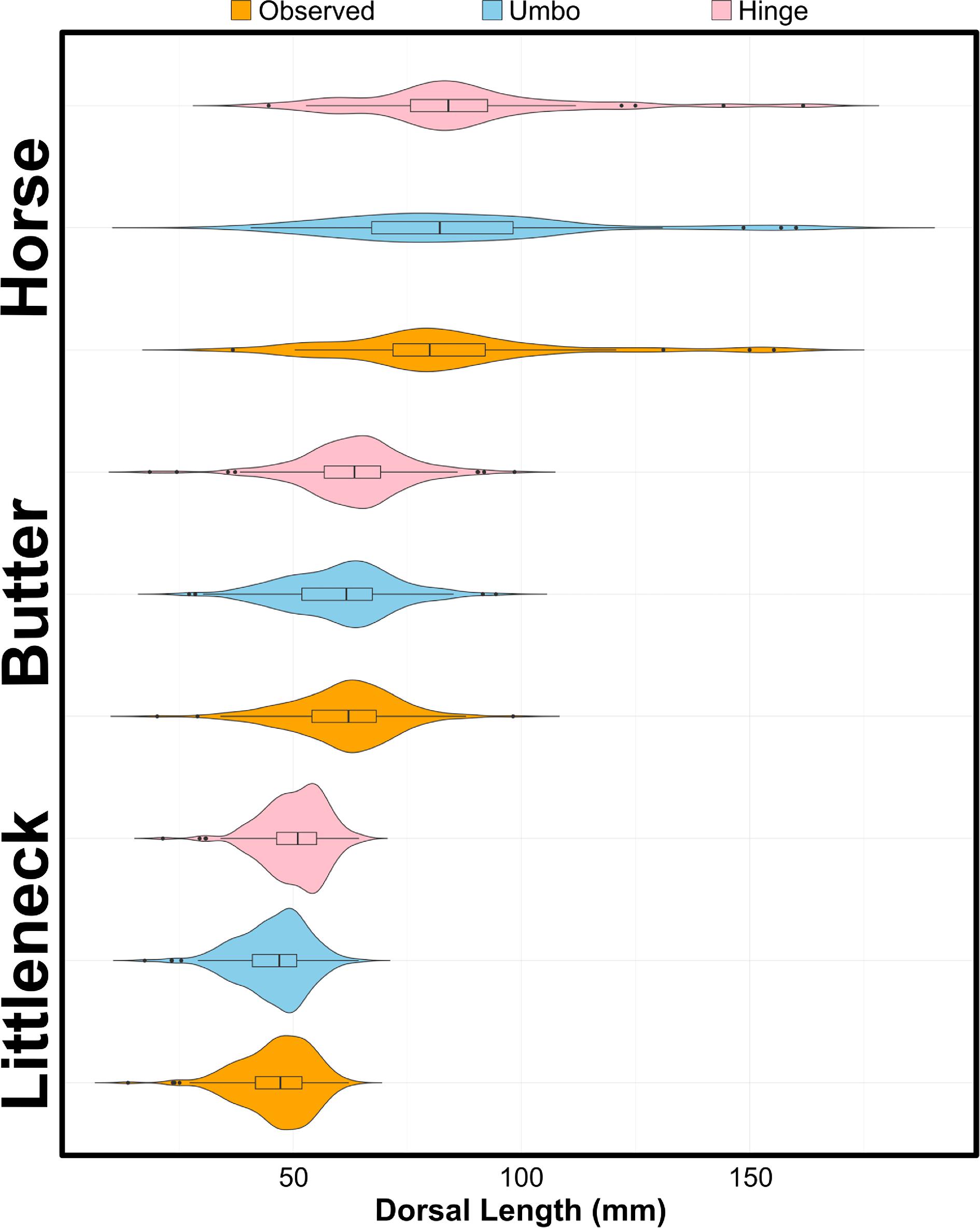
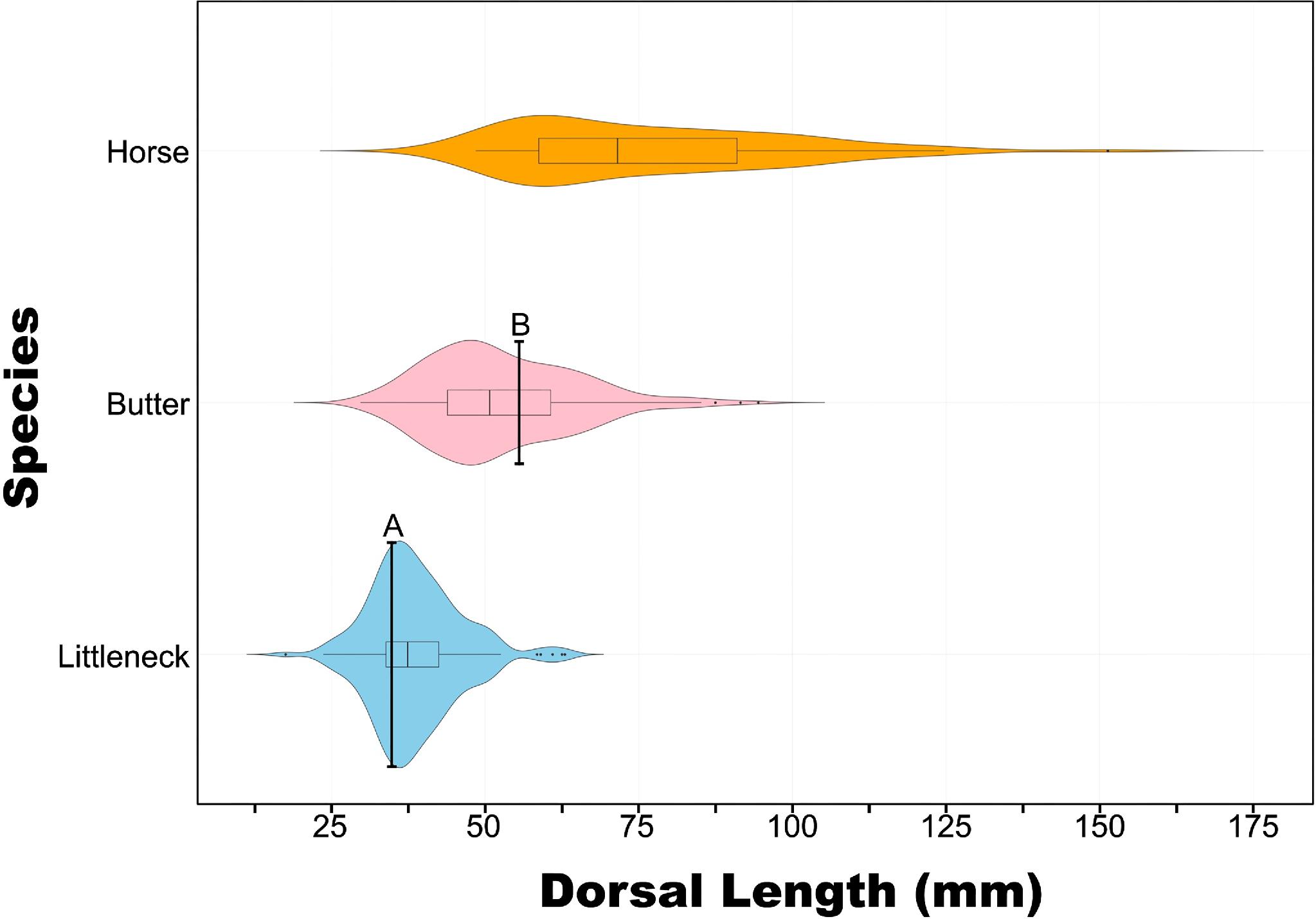
Our examination of archaeological clam length distribution profiles indicates that the median size of ancient clam harvests at
Ka
kma
kimilh closely resembles contemporary size limits established in fisheries management (
Department of Fisheries and Oceans 2023). While ecological processes and stewardship practices, such as clam gardening, can influence the relationship between size-at-age, the
Ka
kma
kimilh size data offer a historically grounded size-at-harvest baseline from which further research can build upon. Given that these size-at-harvest profiles span 3000 years of human use and are broadly comparable to contemporary legal size limits (
Fig. 5), we interpret these findings as evidence of sustained use and as an indication of size-based clam management practices. These findings highlight the resilience of this social–ecological system and support other data documenting Tseshaht peoples’ participation in pre-industrial marine food webs and the role of shellfisheries in fostering community food sovereignty and security (
McKechnie 2015;
Efford 2019;
Hillis et al. 2020;
Slade et al. 2022;
Popken et al. 2023). We anticipate that further research will explore these data over time, including ongoing research in Barkley Sound (
Gustas et al. 2022;
Barclay et al. 2024), and in other coastal regions to better understand the nuances of Indigenous shellfish management practices and the ecological effects of millennia of human-clam relationships.
These findings highlight the applicability of a simple and efficient method for estimating the size-at-harvest of three clam species commonly found in coastal archaeological assemblages. A further strength of these models is the inclusion of clams from a broad geographic range as well as both modern and preindustrial periods, as these data span varied environmental conditions. While we do observe modest regional differences in dorsal length for a given umbo or hinge size, the scaling relationship across species and regions is the same. Given the increased uncertainty associated with intra- and inter-observer error compared to regional variation, we chose to use a reduced model to appeal to a broad range of practitioners working across the coast. Despite more complex multivariate approaches to shellfish size prediction that can achieve greater precision (e.g.,
McFarland et al. 2023), our results suggest that a simple linear model is sufficient to capture a broad, inclusive signal of clam size across the three species examined. Moreover, the accessibility of this method offers future research teams the ability to generate comparable data through a standardized morphometric approach (e.g.,
Fig. 2), while its simplicity provides opportunities to involve people from diverse and nonscientific backgrounds in data collection and analysis. We envision that more complex and regionally specific datasets could be added to test for any deviations, such as growth rates, environmental variability, sea level and climatic changes, predation, and (or) ontogeny.
The strong relationship between umbo thickness and dorsal length provided the most robust predictions and is supported by broader allometric scaling relationships observed across species and taxonomic phyla (e.g.,
West et al. 1999). As the umbo often forms the most robust portion of a shell, has distinct species-specific morphological landmarks, and is less affected by inter-observer error, we recommend researchers focus measurement efforts on the umbo rather than the hinge. Moreover, in archaeological investigations, the umbo is regularly used to estimate the proportional abundance of bivalve taxa (i.e., “Minimum Number of Individuals”) as each umbo is nonrepeatable and can be sided (
Giovas 2009). Indeed, clam size is particularly relevant for quantifying bivalves recovered from small-volume, fine-screened archaeological “bulk” or “column” samples, as this can enable counts of individual clams per liter of sediment. While beyond the scope of this current study, we envision future research developing shell length-to-weight conversion factors (e.g.,
Bradbury et al. 2005;
Barber et al. 2012) as a first step in exploring proportional clam biomass as well as generating estimates of biomass represented in archaeological deposits (e.g., clam meat weight per liter of sediment). With such data, researchers will then be able to scale up analyses from small volume samples to investigate shifts in harvested shellfish biomass over time (e.g., harvested clam biomass per century) and contribute to research on past human diet, demography, and the ecosystem effects of millennia of Indigenous harvests.
While numerous environmental and cultural factors can influence clam growth, resulting in clams of the same age exhibiting variability in size, the allometric scaling relationship we observe between morphological features provides a general basis for establishing size as a useful metric irrespective of age. Indeed, the close and predictable relationship between dorsal length and the umbo and hinge makes this a practical approach for determining size-at-harvest. We also recognize that examining size alone may not accurately capture the frequency at which a population is harvested (i.e., harvesting intensity). Yet, such an approach has the potential to be combined with size-at-age methods and sclerochronological studies involving geochemical analyses, which often rely on fragmentary shell valves lacking size estimates, to help refine the relationship between size and age. Assessing any deviations from a typical allometric growth relationship could also be used to identify differences in environmental conditions between populations. Researchers may also opt to utilize this approach as a stand-alone method to better understand the size-selective preferences and protocols of Indigenous harvesters, the relative contribution of clams to ancient foodways (i.e., harvested clam biomass), and the influence of nonhuman predation pressure (e.g., otters, sea birds, crabs, etc.), among other applications.
The development of a simple linear regression-based methodology for evaluating size-at-harvest profiles from fragmentary clamshells can deepen contemporary knowledge about the enduring and millennia-old relationships between shellfish and Indigenous peoples across the Northwest Coast. Our approach addresses a methodological gap as archaeologists increasingly recognize the role of clam gardens and traditional management practices in supporting Indigenous food systems (e.g.,
Jackley et al. 2016). Yet, much of recent research on archaeological clam shells has focused on more complex methods, such as sclerochronological geochemistry (e.g.,
Burchell et al. 2013;
Schöne et al. 2020) and size-at-age relationships (
Toniello et al. 2019). Many of these studies rely on small-volume sediment samples with high degrees of fragmentation and, accordingly, often lack more basic information on shell sizes. Earlier studies that do report shell size data (e.g.,
Wessen 1982;
Coupland et al. 2003) occurred at large-scale multi-year excavation projects. Therefore, size-at-harvest represents a neglected field of research that can contribute to efforts aimed at revitalizing Indigenous food systems today. We envision this simple measurement-based approach as a suitable first step for generating abundant size-at-harvest data across broad spatial, temporal, or environmental scales. Other research efforts focused on characterizing size-at-age may also find utility in having a standardized approach for measuring the various morphological attributes of clamshell valves. Ultimately, such data can help extend perspective on ecological integrity and ecosystem recovery trajectories (
Wickham et al. 2022;
Gann et al. 2019;
Reeder-Myers et al. 2022) and better understand the historical conditions under which humans and clams persisted over millennia, which is essential for establishing appropriate and culturally relevant conservation objectives today.