1. Introduction
One of the most prevalent chronic liver illnesses since 2000 is nonalcoholic fatty liver disease (NAFLD) (
Chalasani et al. 2012;
Sattar et al. 2014). The incidence of NAFLD is as high as 20%–40% around the world (
Rinella 2015), and recently the prevalence of NAFLD in China reached 29.2% (
Zhou et al. 2019). Its morbidity and mortality are affected by cirrhosis, liver fibrosis, and nonalcoholic steatohepatitis (NASH), all of which are brought on by NAFLD (
Singh et al. 2015). As opposed to alcoholism or other conditions that lead to hepatic steatosis, NAFLD is featured by a significant accumulation of lipids in the liver cells (
Fan et al. 2019). NAFLD is now regarded as the metabolic syndrome’s forerunner (
Lonardo et al. 2015;
Ballestri et al. 2016a), and can greatly raise the risk of heart disease (
Targher et al. 2010;
Targher et al. 2016), type 2 diabetes (
Ballestri et al. 2016b), and other diseases. Its pathogenesis and influencing factors are complex. Many factors influence their clinical presentation and disease progression, including metabolism, dietary habits, race, immunity, intestinal flora, genetic susceptibility, and other factors (
Rong et al. 2023).
All around the world, particularly in underdeveloped nations,
Helicobacter pylori (
H. pylori) is quite prevalent (
Wu et al. 2005;
Shehata et al. 2017;
Alboraie et al. 2019). Additionally, elderly individuals have a higher probability than teenagers to get this infection (
Cizginer 2014;
Abd-Elsalam et al. 2016). One of the most prevalent bacteria causing disorders of the human digestive tract is
H. pylori. Some foreign scholars proposed the transformation from nonatrophic to atrophic gastritis, intestinal metaplasia, hyperplasia, and stomach carcinoma after
H. pylori infection as early as 1992, and there is also a close relationship between
H. pylori and extra gastrointestinal disorders, such as liver tumors and abnormal lipid metabolism (
Gravina et al. 2018). A study showed that
H. pylori infection may have a role in the emergence of NAFLD, and its elimination may be crucial to the therapy of this condition (
Polyzos et al. 2013). Another Japanese investigation revealed that
H. pylori infection is a separate risk factor for NAFLD (
Takuma 2011). The reported mechanism of action of H. pylori infection in NAFLD may be related to inflammation, metabolic effects, changes in intestinal permeability, and the gut microbiome (
Mavilia-Scranton et al. 2023). It is possible that each study object or research method, sample size is different, resulting in different results. It has not been thoroughly investigated how
H. pylori affects NAFLD.
More researchers domestically and internationally have reported the connection between
H. pylori and the prevalence of NAFLD, but the sample size of the study population is small, and the research results are different. Therefore, in 2013 the study team performed a cross-sectional survey on all middle-aged and senior employees at a petrochemical firm in Zhenhai District, Ningbo, to further examine the connection between
H. pylori and NAFLD. It was shown that the group with
H. pylori had a higher rate of NAFLD detection, but no significant correlation was found in the logistic regression analysis (
Chen et al. 2016), but in the elderly in the same research object, a risk factor for NAFLD was discovered to be
H. pylori infection (
Chen et al. 2017b). Considering that the influence of
H. pylori infection of NAFLD may have a time accumulation effect, we used a cohort study to collect non-NAFLD as the research object. According to whether
H. pylori infection or not, we divided them into two groups to track the incidence of NAFLD in the past 4 years, so as to explore whether NAFLD and
H. pylori have a causal association and the degree of association, and offer a fresh strategy for treating and preventing NAFLD.
2. Subjects and methods
1. Determination of research objects
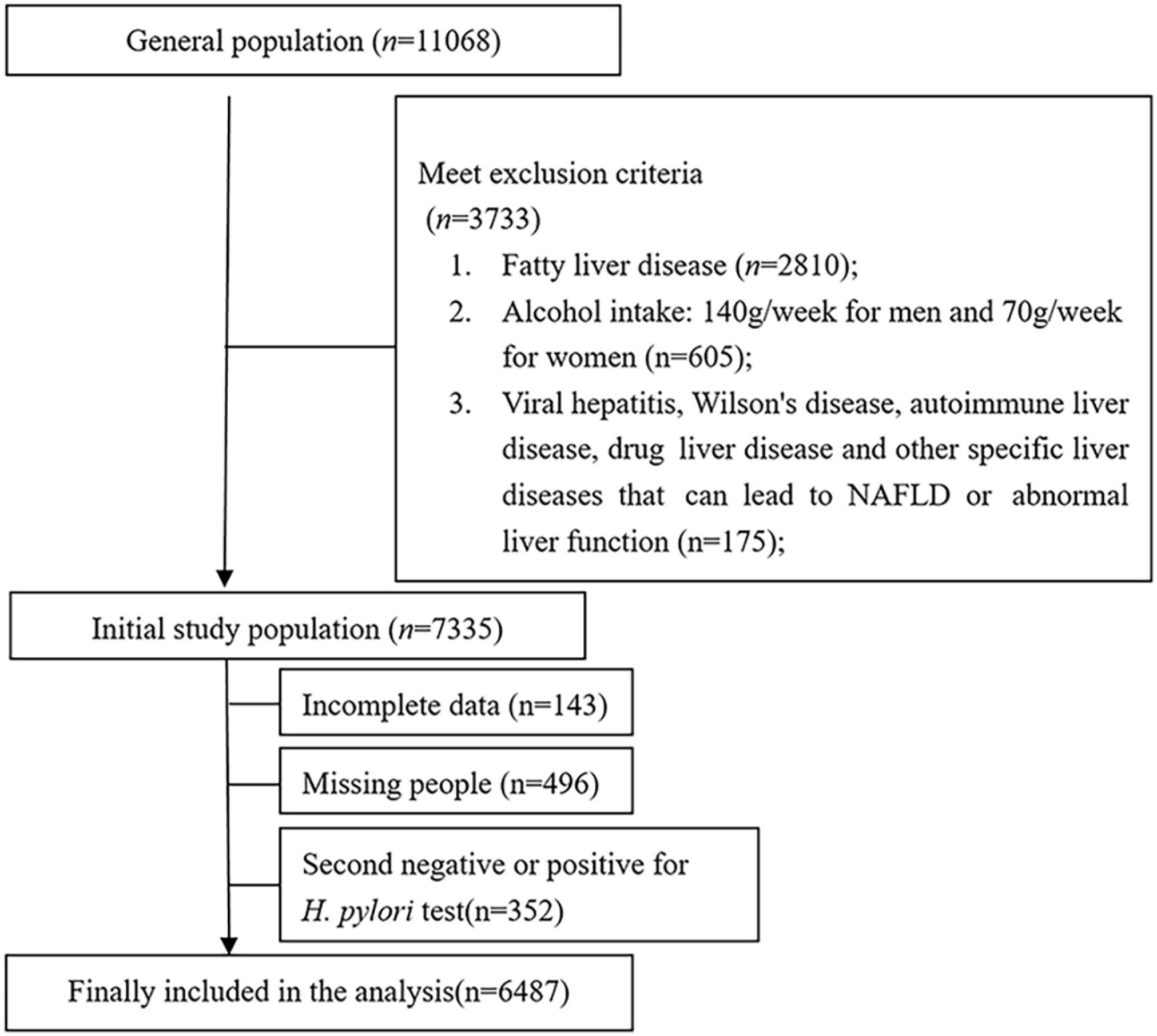
The employed and retired members who were selected as the basic research cohort had a physical checkup at the Zhenhai Refining and Chemical Hospital of Ningbo City between March 2013 and November 2013, with a total of 11 068 patients aged 22–89 years. The exclusion criteria were: fatty liver (n = 2810) had been diagnosed by B-ultrasound in the middle and upper abdomen, drug-induced liver damage, viral hepatitis, autoimmune liver disease, hepatolenticular degeneration, as well as other certain liver conditions that may cause NAFLD or impaired liver function; subjects with long-term drinking history and the equivalent amount of alcohol is more than 140 g per week for males and more than 70 g per week for females; patients with gastric cancer and peptic ulcer; used bismuth, glucocorticoids, proton pump inhibitors, nonsteroidal anti-inflammatory medications, or antibacterial drugs in recent 4 weeks; previous H. pylori eradication treatment; have a history of gastrointestinal surgery. Those who sign to give up the benefits of this physical examination and whose data are incomplete and cannot be analyzed due to various reasons will have a physical examination once a year for all subjects, which is consistent with the baseline examination items and methods. The exclusion criteria include the items that have undergone H. pylori eradication treatment. The Ethics Committee gave its approval to this study (approval No.: zhenlianyi (2012) No. 2), and the informed consent form was signed by every subject.
2. General data collection
After the short-term training for the investigators, the abovementioned physical examination persons were given a unified questionnaire and related examinations. The contents of the questionnaire included general information, including age, gender, drinking, and smoking history, whether they were taking liver protection drugs, whether they were undergoing H. pylori eradication treatment, etc. Diabetes, liver illness, gastrointestinal disease, hypertension, hyperlipidemia, and recent medication use are all included in the patient’s medical history. Examination method: the patient’s weight, height, and waist size were all measured in the morning on an empty stomach using a standard method, and their systolic and diastolic blood pressure was determined by sitting for 5 min. When there is an anomaly for the first measurement, we measure the second time and record the average value of the measurement.
3. Data collection methods
Ten mL elbow vein blood was drawn on an empty stomach for the detection of serum biochemical indicators. After that, two chief imaging specialists used two ultrasonic diagnostic instruments of the same model (Philips iu22 color ultrasonic diagnostic equipment with a convex array probe and a frequency of 2–6 MHz) to operate and publish a report. The results of the same subject were tested by two observers for consistency, and the Kappa value was greater than 0.8. The radiologist was unaware of the history of the subject and the study. C-13 urea marker expiratory samples were detected with “HCBT-01” expiratory test tester (Shenzhen Zhonghe Haidewei Biotechnology Co., Ltd.) on an empty stomach. These are the precise steps: (1) baseline expiratory samples collection; (2) about 50 mL drinking water, 75 mg c-13 urea capsules; (3) breath samples were collected and measured after sitting for 30 min; (4) the measurement of breath samples prior to and after ingesting C13 urea capsules was performed using a C-13 breath mass spectrometer. The outcomes were presented as delta-over-baseline (DOB).
4. Diagnostic criteria
For NAFLD diagnosis, refer to the B-ultrasound diagnostic criteria in the diagnosis and treatment guidelines for nonalcoholic fatty liver disease (2010 Revision) issued by the hepatology branch of the Chinese Medical Association in 2010 (
Jian-gao and Chinese Liver Disease Association 2010). According to this diagnostic criteria, the subjects were split into control group whose B-ultrasound examination did not fulfill the NAFLD criteria and NAFLD group. The positive criteria of
H. pylori infection were DOB ≥ 4.0.
5. Statistical methods
For data processing, SPSS18.0 statistical software was employed. The data of continuous variables were expressed in (±s) or m (P25, p75), and chi-square test was used for comparisons with categorical variables; Mann–Whitney U test or t-test was used for group comparisons; the Cox regression analysis was employed to discover the NAFLD risk factors. P < 0.05 indicated that the results were statistically different.
4. Discussion
The association between
H. pylori infection and the development of NAFLD has been reported in several previous literatures, but with inconsistent results (
Takuma 2011;
Chen et al. 2016;
Fan et al. 2018). Thus, this research collected the relevant data of NAFLD and
H. pylori in the physical examination participants and followed up for 4 years. To investigate the connection between NAFLD and
H. pylori infection, as well as the potential risk factors that may influence the incidence of both. The findings of this study imply that
H. pylori infection may be connected to NAFLD. First, the prevalence of NAFLD was significantly higher in the
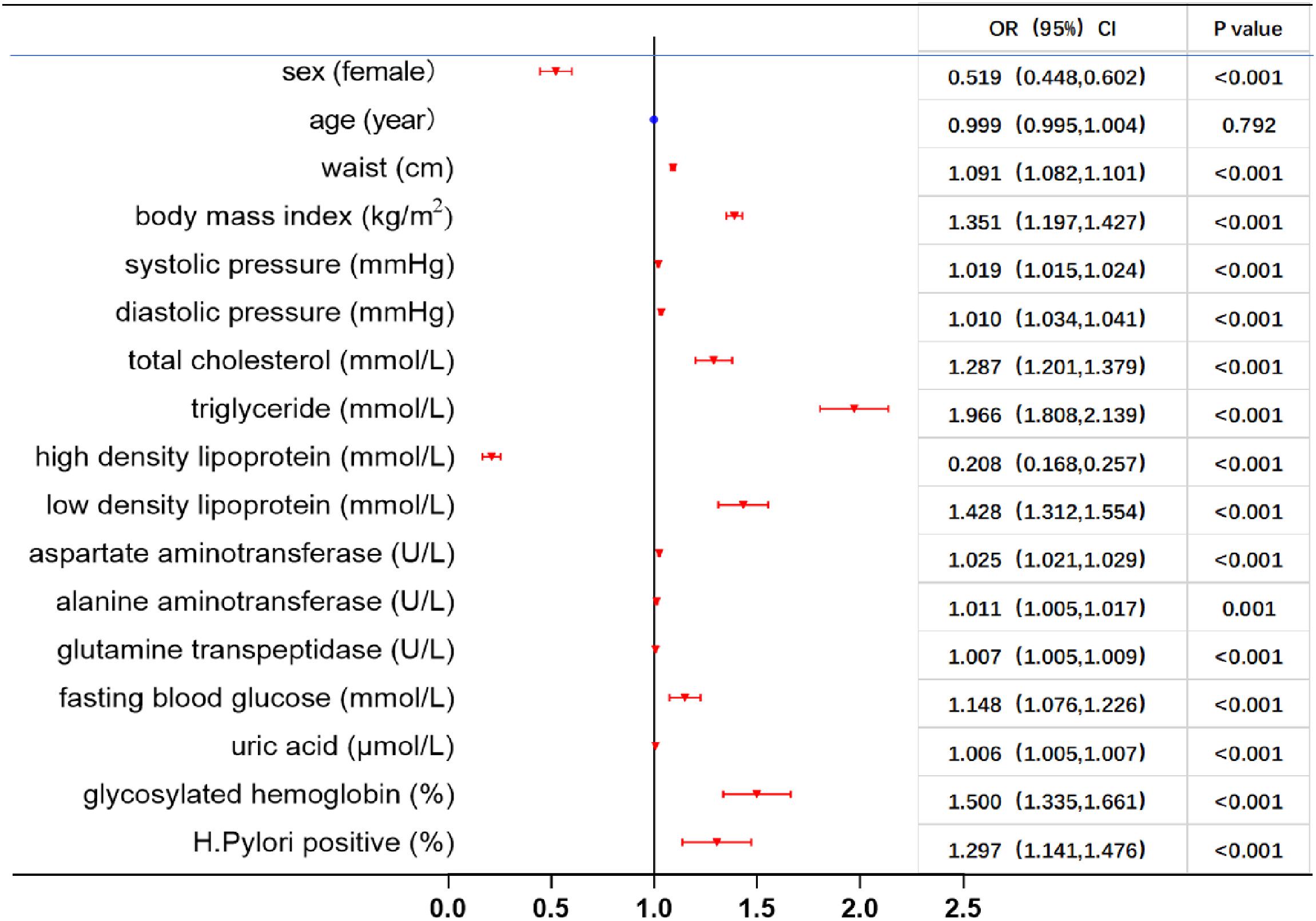
H. pylori positive group than in the negative group. Second, compared to the negative group, the positive group had greater levels of waist, total cholesterol, systolic blood pressure, BMI, and low density lipoprotein. Third, multivariate Cox regression analysis revealed that
H. pylori infection was substantially related to NAFLD risk variables.
Common gastrointestinal problems can be brought on by
H. pylori infection, which is connected to extra-gastrointestinal illnesses, such as NAFLD, diabetes, metabolic syndrome, allergic asthma, etc. (
Chen et al. 2017a;
Polyzos et al. 2019). Through the action of chronic inflammatory mediators,
H. pylori infection may enhance the incidence of NAFLD. According to studies, associated inflammatory chemicals such as interleukin-6, tumor necrosis factor, and hypersensitive C-reactive protein can be expressed more frequently as a result of the chronic inflammatory response brought on by
H. pylori (
Yildirim et al. 2016). A number of kinases in the body will be activated by these inflammatory cytokines. These enzymes eventually trigger insulin resistance by increasing serine phosphorylation or preventing the tyrosine in the insulin receptor substrate from autophosphorylation (
Esser et al. 2014), and insulin resistance is one of the important pathogenic mechanisms of NAFLD. Additionally, this study discovered that the
H. pylori positive group had higher waist circumference, total cholesterol, BMI, and low density lipoprotein levels than the
H. pylori negative group had, while high-density lipoprotein levels were lower than those in the
H. pylori negative group, which was in line with the findings of
Kim et al. (2017). These findings indicate that
H. pylori infection may induce NAFLD through mediating abnormal changes in blood lipid profile and inflammatory factors.
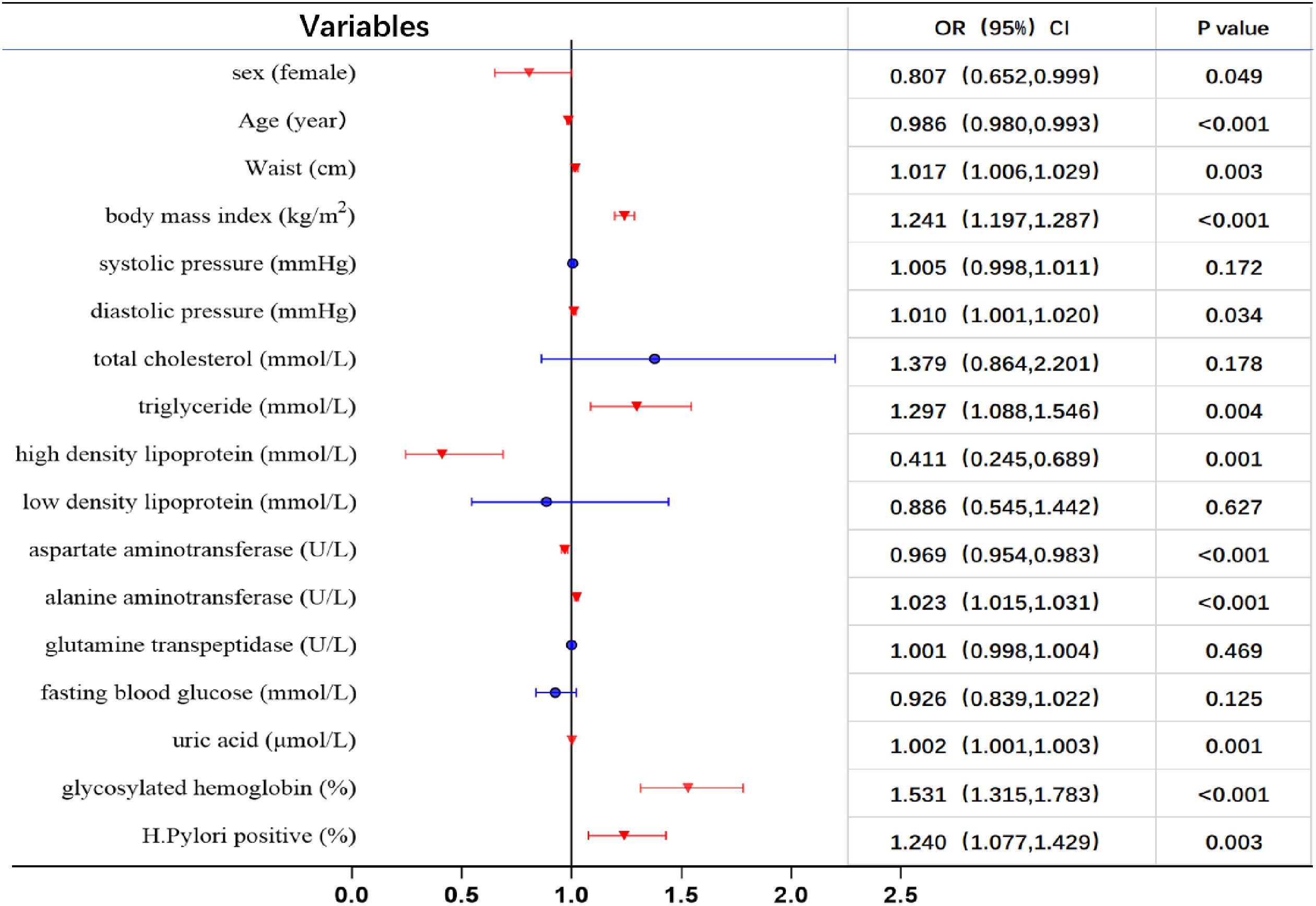
Throughout the study’s four-year follow-up, a total of 1160 new cases of NAFLD were found. Overall, the incidence density of NAFLD was 4.23/100 person-years. Over the 4 years, there was a statistically significant difference in incidence density between the two groups, with a substantially higher incidence of new cases of NAFLD the
H. pylori positive group than in the
H. pylori negative group. Total cholesterol, triglycerides, low density lipoprotein cholesterol, waist circumference, BMI, systolic and diastolic blood pressure, aspartate aminotransferase, alanine aminotransferase, glutamine transpeptidase, fasting blood glucose, uric acid, and glycosylated hemoglobin were substantially higher in new NAFLD patients than those in the non-NAFLD group, whereas high density lipoprotein was lower than in the non-NAFLD group. All components of the metabolic syndrome are strongly associated with NAFLD, which is consistent with the reports of foreign researchers (
Cariou et al. 2021). An independent risk factor for NAFLD was
H. pylori infection, according to Cox proportional hazards model analysis. After adjusting for confounding variables as gender, age, and elements of the metabolic syndrome, the risk of NAFLD was 1.284 times higher than in the
H. pylori negative group.
H. pylori infection has been associated with NAFLD as a risk factor in many studies (
Chen et al. 2016;
Jiang et al. 2019;
Abo-Amer et al. 2020).
Ning et al. (2019) conducted a meta-analysis of 12 studies involving 27 400 NAFLD patients and 60 347 controls and discovered a risk of
H. pylori infection of 1.36 (95% CI: 1.22–1.53,
I = 89.6%,
P = 0.000) in uninfected individuals, which is consistent with the results of the present study. For example,
Mohammadifard et al. (2019) determined whether
H. pylori infection was present by ELISA detection of
H. pylori antigen and
H. pylori igg in 65 cases in the stool of case and control group. No statistical differences were found between the two cases. However, there are some conflicting results, such as two cross-sectional studies in Japan (
Okushin et al. 2015) and South Korea (
Baeg 2016) in recent years, which suggested that
H. pylori infection is not connected to NAFLD and may not be a risk factor for NAFLD. Considering regional and diagnostic differences and limited sample sizes, future clinical trials with large sample sizes are needed to accurately determine the association between
H. pylori infection and NAFLD.
In addition, the study failed to evaluate the time interval and frequency of the four examinations in detail, did not assess the status of H. pylori infection during the follow-up, and NAFLD was diagnosed by b-ultrasound rather than liver biopsy, which may also affect the reliability of the results to some extent. However, since all the subjects in this physical examination were voluntary and the follow-up rate was high, the results of this study still have a certain reference value.