Introduction
Biodiversity is under threat from anthropogenic stressors, including resource extraction, invasive species, and climate change (
Martin et al. 2020). A key pillar to combat biodiversity loss is area-based conservation measures (e.g., marine protected areas (MPAs) and other effective conservation measures (OECMs)) that may serve as refuges from resource extraction or development. These areas not only provide direct biodiversity conservation benefits through the regulation of activities (e.g., fishing) within defined spatial boundaries but may also confer indirect benefits through the prevention of habitat loss and degradation. Accordingly, international agreements have been established to protect 30% of global habitats by 2030 (UN Environment Program 2022
1).
Unfortunately, without review and flexibility, area-based conservation measures may be poorly suited to deal with stressors such as climate change that progressively alter ocean conditions in relation to protected area boundaries (
Bruno et al. 2018). This is particularly relevant for areas delineated based on species-specific conservation objectives. As climate change alters suitable habitat and the distribution of focal species relative to protected areas, climate-adaptive management will be key to effective conservation efforts (
Pentz and Klenk 2023).
Reacting to ecosystem changes is one component of adaptive management, but there is an increasingly recognized need for proactive planning based on anticipated change. Vulnerability assessments of species to projected climate change impacts have been proposed as one tool to inform proactive planning of conservation areas (
Bryndum-Buchholz et al. 2022). To do this, however, we must understand how species will be redistributed with climate change, the effects of which are not uniform across regions or among species (
Cheung et al. 2009;
Dahlke et al. 2020). Furthermore, within species, certain life history stages may be more sensitive to environmental change. Identifying the vulnerabilities to climate change of focal conservation species in protected areas will thus require an understanding of both projected climate trends at a local scale as well as a mechanistic understanding of how a changing environment will influence the fortunes of those species (
Pentz and Klenk 2023).
Within the Newfoundland and Labrador Bioregion of Canada, a combination of MPAs and OECMs has been established, each with their own set of conservation objectives. Atlantic cod, for example, is an ecologically and culturally important species listed as a conservation objective for multiple protected areas within the region. Along the eastern Newfoundland and southeastern Labrador shelf, the Funk Island Deep Closure and Hawke Channel Closure prohibit bottom trawl, gillnet, and longline fisheries to conserve Atlantic cod as well as benthic habitat (while the Gilbert Bay MPA confers protection of Gilbert Bay cod, this is a unique coastal subpopulation of Atlantic cod that is beyond the scope of our current paper). Other closures may protect the habitats and species of the demersal zone more generally (
Table 1).
Understanding how climate change may threaten the effectiveness of regional closures through altered abiotic conditions is important as many of their conservation objectives are species- or habitat-specific and delineated based on current distributions.
Cote et al. (2021) projected northward shifts of cod species over time but also mismatches in thermal suitability of two different life stages: eggs and juveniles. While northward shifts were modest, there were notable changes expected in the total area of suitable habitat since latitudinal shifts pushed Atlantic cod into narrower shelf habitats further north. However, since protected areas are distributed across the region, there is potential that they, as a network, can mitigate the negative consequences of species shifts (
Bryndum-Buchholz et al. 2022). Specifically, while poleward shifts may push Atlantic cod out of currently occupied protected areas, a well-designed network would have alternate protected habitats available to receive displaced populations. It remains unclear, however, whether the current network will maintain similar areas and/or quality of protected habitats as climate change progresses.
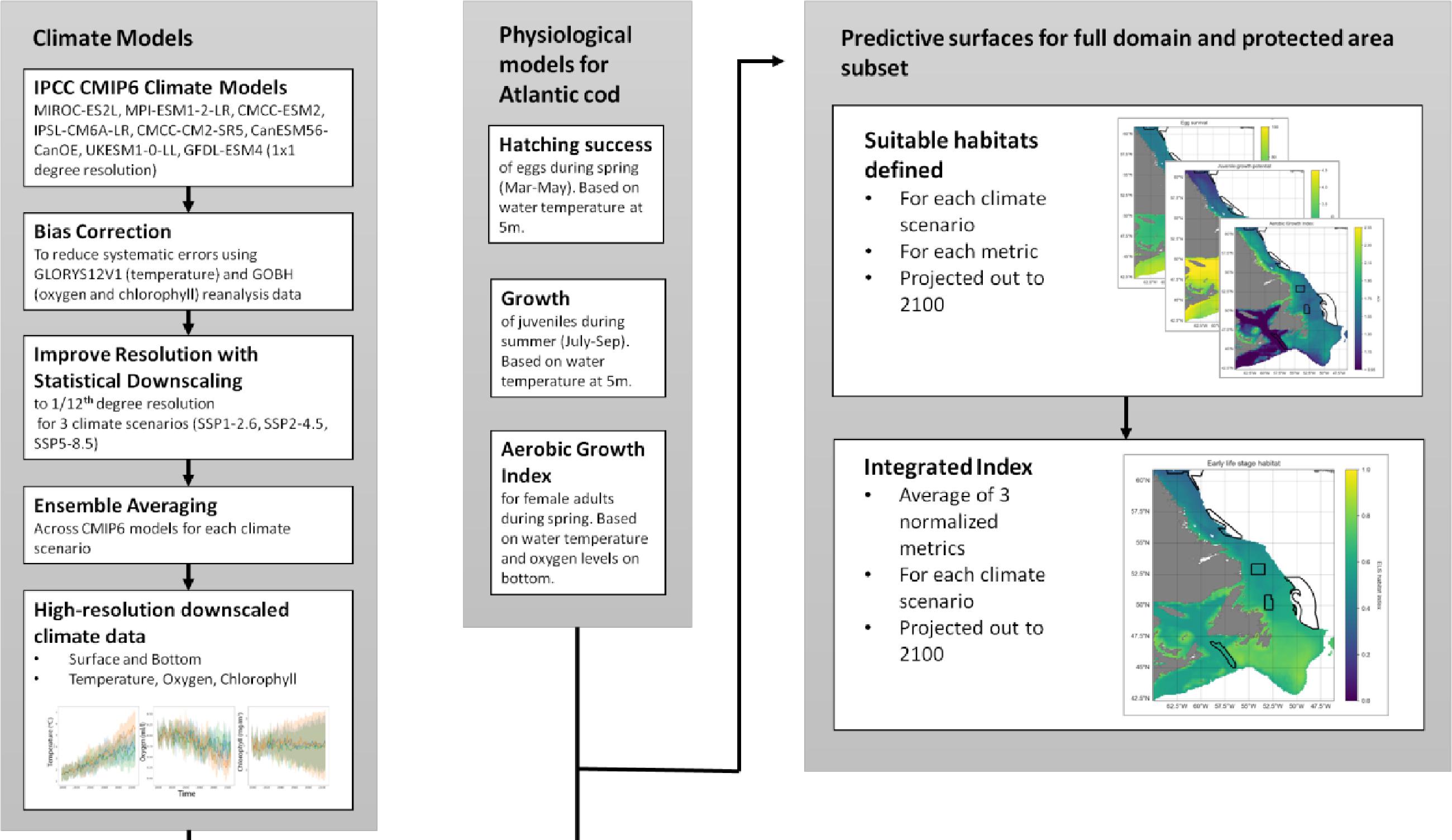
Here, we combine high resolution downscaled climate predictions with mechanistic models for three different life stages of Atlantic cod (eggs, juveniles, and spawning adults) to examine how habitat suitability in terms of temperature and oxygen may be altered by climate change off the coast of Newfoundland and Labrador in the future (the Northwest Atlantic Fisheries Organization (NAFO) 2J3KL cod stock). Mechanistic projections are particularly useful because they can capture nonlinear population constraints on a variety of life stages and/or habitats that may not be captured by the surveys that inform correlative approaches (
Araujo and Guisan 2006;
Cote et al. 2021). With these predictions, we evaluate how habitat suitability may be altered for the larger Newfoundland and Labrador region, as well as within individual protected areas, to highlight areas where conditions may improve or be severely impacted in the future. By examining the impacts of climate change on multiple life stages of Atlantic cod in isolation and in combination, we can identify potential habitat suitability bottlenecks in space and time. Such information will be vital to predicting the efficacy of protected areas for biodiversity objectives, assessing whether networks can mitigate the shortcomings of individual protected areas in a changing world, identifying climate refugia for new protected areas, and prioritizing monitoring efforts.
Methods
A summary of our methods is shown conceptually in
Fig. 1. Specific methods are detailed below.
Statistical climate downscaling
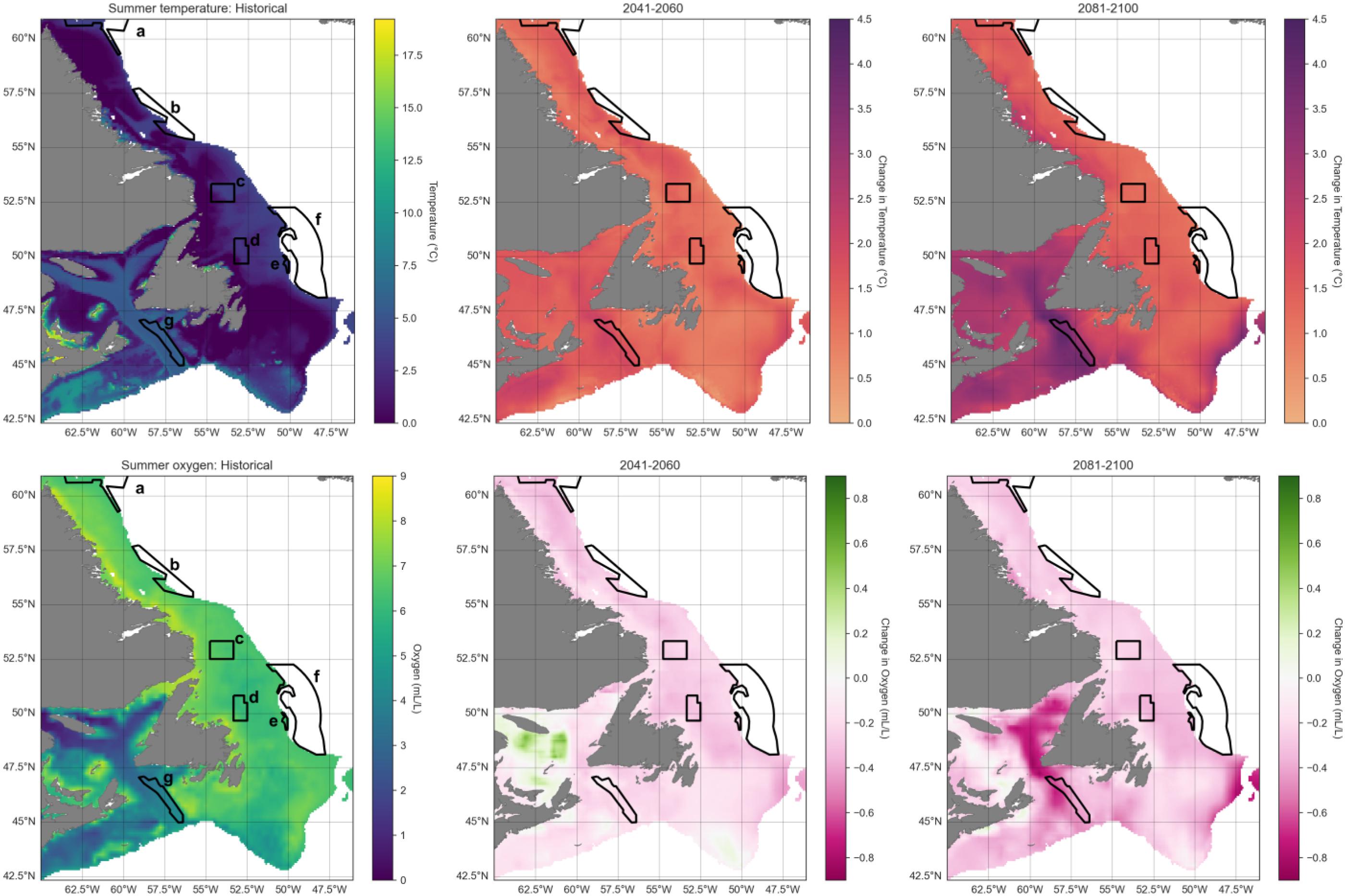
The Intergovernmental Panel on Climate Change (IPCC) report relies heavily on the Coupled Model Intercomparison Project (CMIP) to simulate future climate scenarios based on different emission scenarios. Although the CMIP models cover the atmosphere, land, and ocean on a global scale, their relatively coarse resolution (1 × 1-degree longitude–latitude in the ocean) does not capture the details of the marine coastal domain.
To generate regional climate projections, we applied statistical bias-correction (BC) and downscaling (SD) techniques to global CMIP6 projections. The BC method reduces systematic errors in the climate data by aligning modeled and observed values during a specific control period. Meanwhile, the SD method establishes an empirical relationship between large-scale climate variables and historical data, which is then used to project future climate at local scales.
The bias-correction and statistical downscaling (BCSD) method is based on the approach described by
Lange (2019), but instead uses detrended quantile mapping for BC and SD. Further details and validation of the methodology is described in
Kristiansen et al. (2024). Our approach preserves trends across quantiles, ensures a clear separation between bias adjustment and SD, and provides robust BC for extreme values. The BCSD process consists of two steps: (i) BC of CMIP6 data at their original resolution and (ii) SD of the bias-corrected data to a 1/12th-degree resolution grid. For the BCSD presented here, we use the GLORYS12V1 global reanalysis (
Drévillon et al. 2021;
Drévillon, Fernandez, and Lellouche 2021) as “observations” for physical variables, and we use the Global Ocean Biogeochemistry Hindcast (GOBH;
Perruche et al. 2019) for biological variables (oxygen, chlorophyll). The GOBH model, as described by
Perruche et al. (2019), employs the PISCES model for biogeochemistry and a non-assimilative version of GLORYS for physics. GLORYS12V1, which assimilates various types of historical data from 1993 to 2020, is a state-of-the-art model for hydrodynamics and biogeochemistry. The GLORYS model outputs (e.g., temperature, salinity) at 1/12th-degree resolution, while oxygen and chlorophyll are provided at 1/4-degree resolution. To enable dataset combination, the biogeochemical variables were interpolated and extrapolated to the 1/12th-degree grid using the Earth System Modeling Framework. The final downscaled climate projections for the Newfoundland and Labrador coastal domains are on a 1/12th-degree grid.
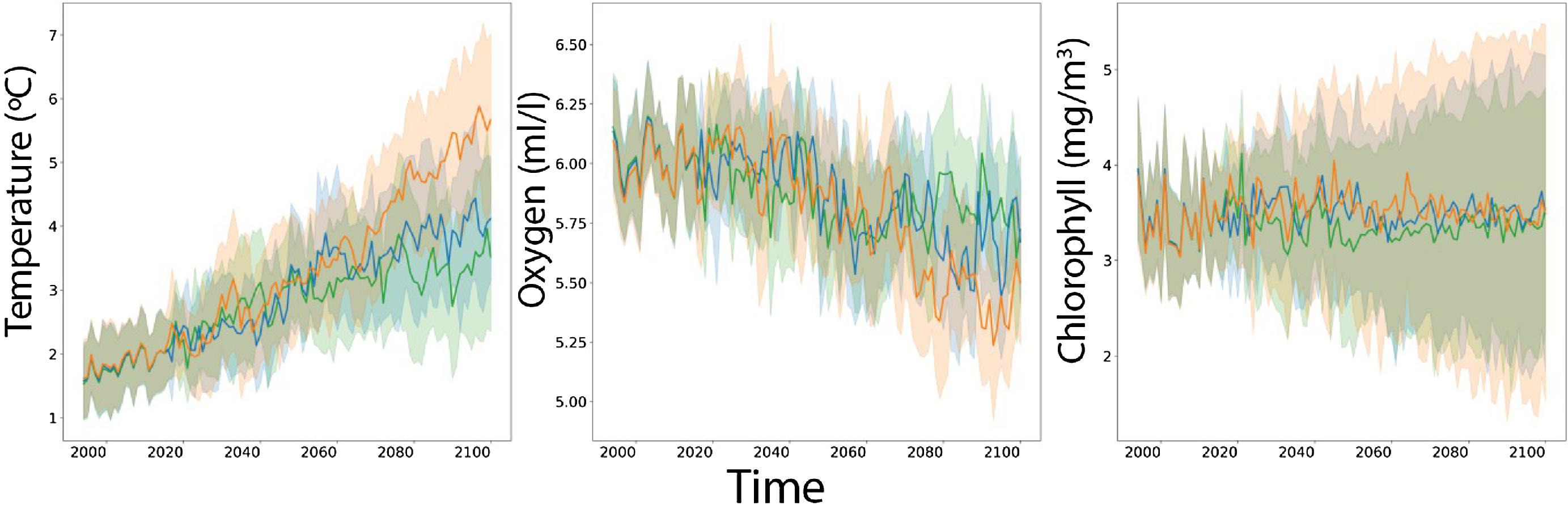
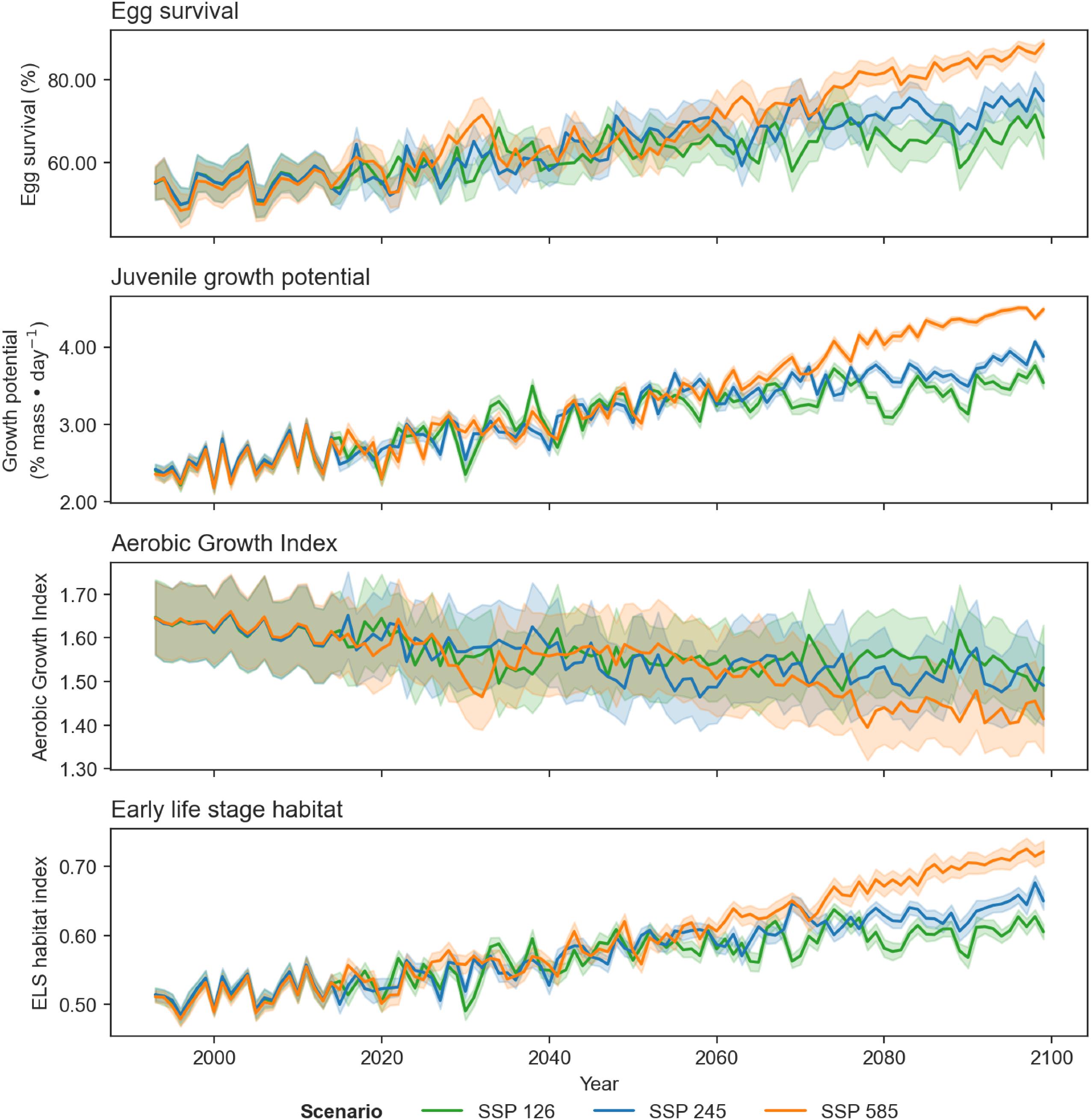
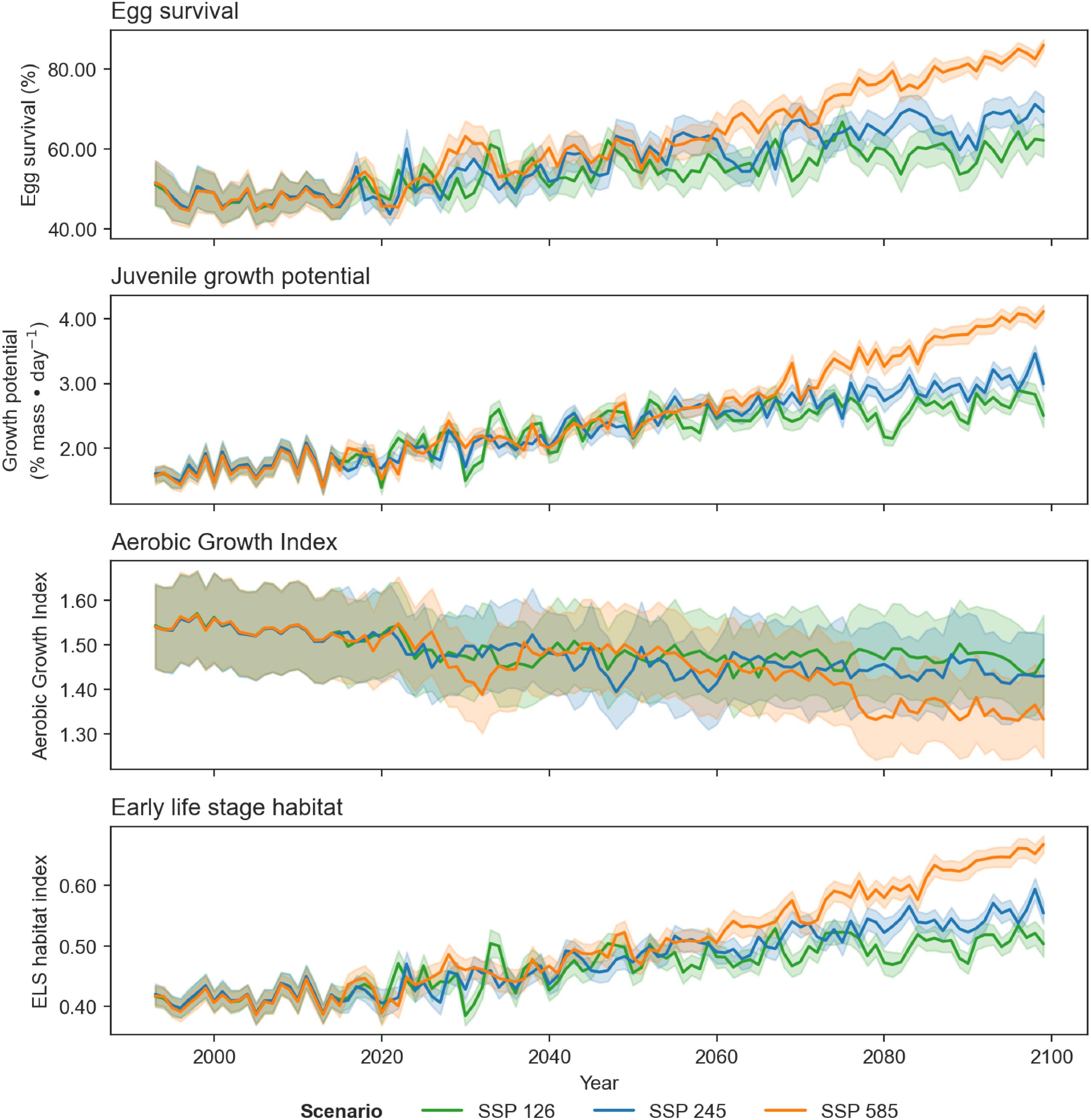
The SD was conducted for the Newfoundland and Labrador coastal domain for a range of CMIP6 climate models MIROC-ES2L, MPI-ESM1-2-LR, CMCC-ESM2, IPSL-CM6A-LR, CMCC-CM2-SR5, CanESM56-CanOE, UKESM1-0-LL, and GFDL-ESM4 (not all variables like chlorophyll and oxygen are available for all models). Downscaling was conducted for three different climate scenarios SSP1-2.6 (“sustainable development”), SSP2-4.5 (“middle of the road”), and SSP5-8.5 (“business as usual”). The wide range of CMIP6 models enabled us to calculate an ensemble average of ocean temperature, salinity, pressure, oxygen, and chlorophyll at the surface (5 m) and bottom depths.
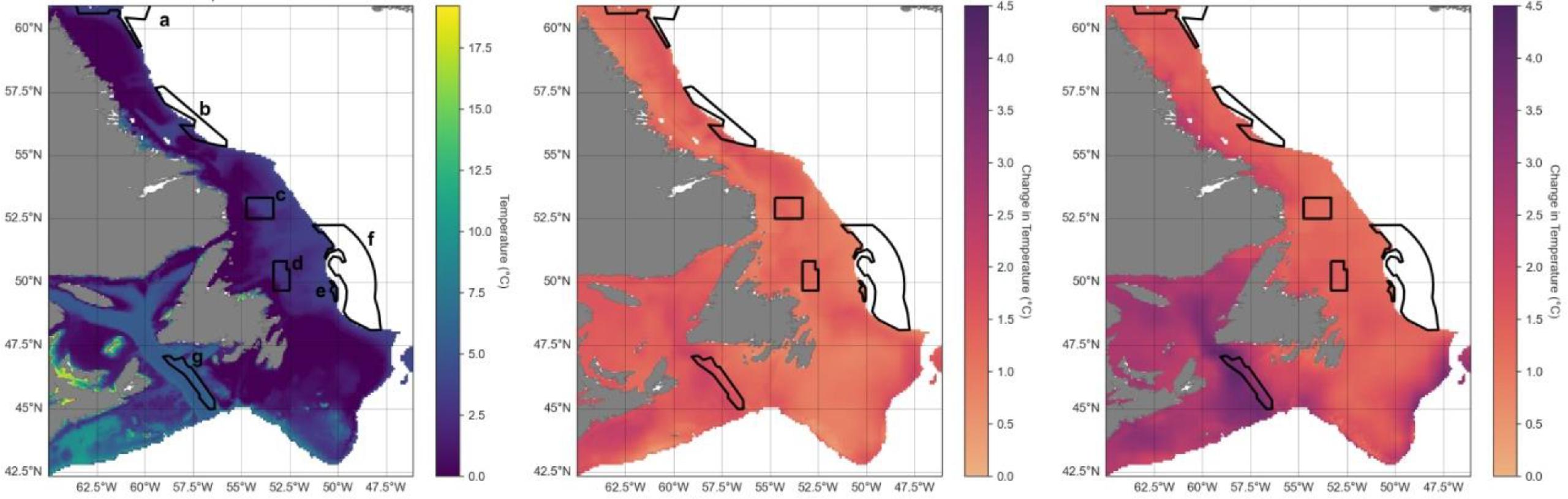
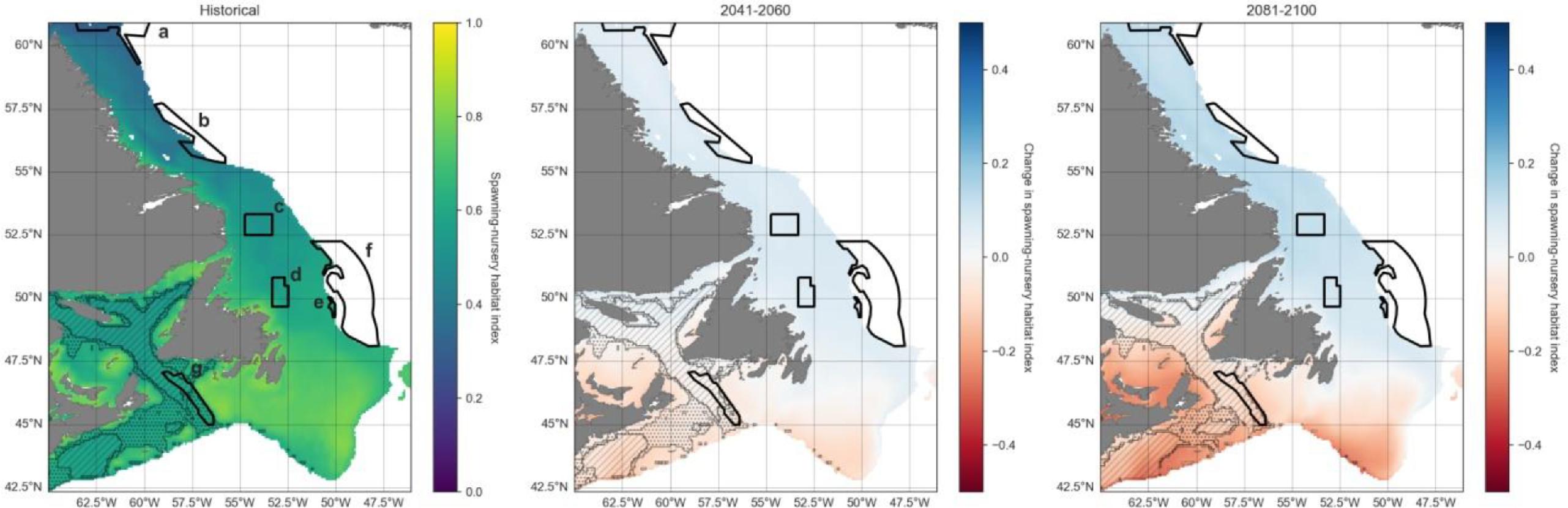
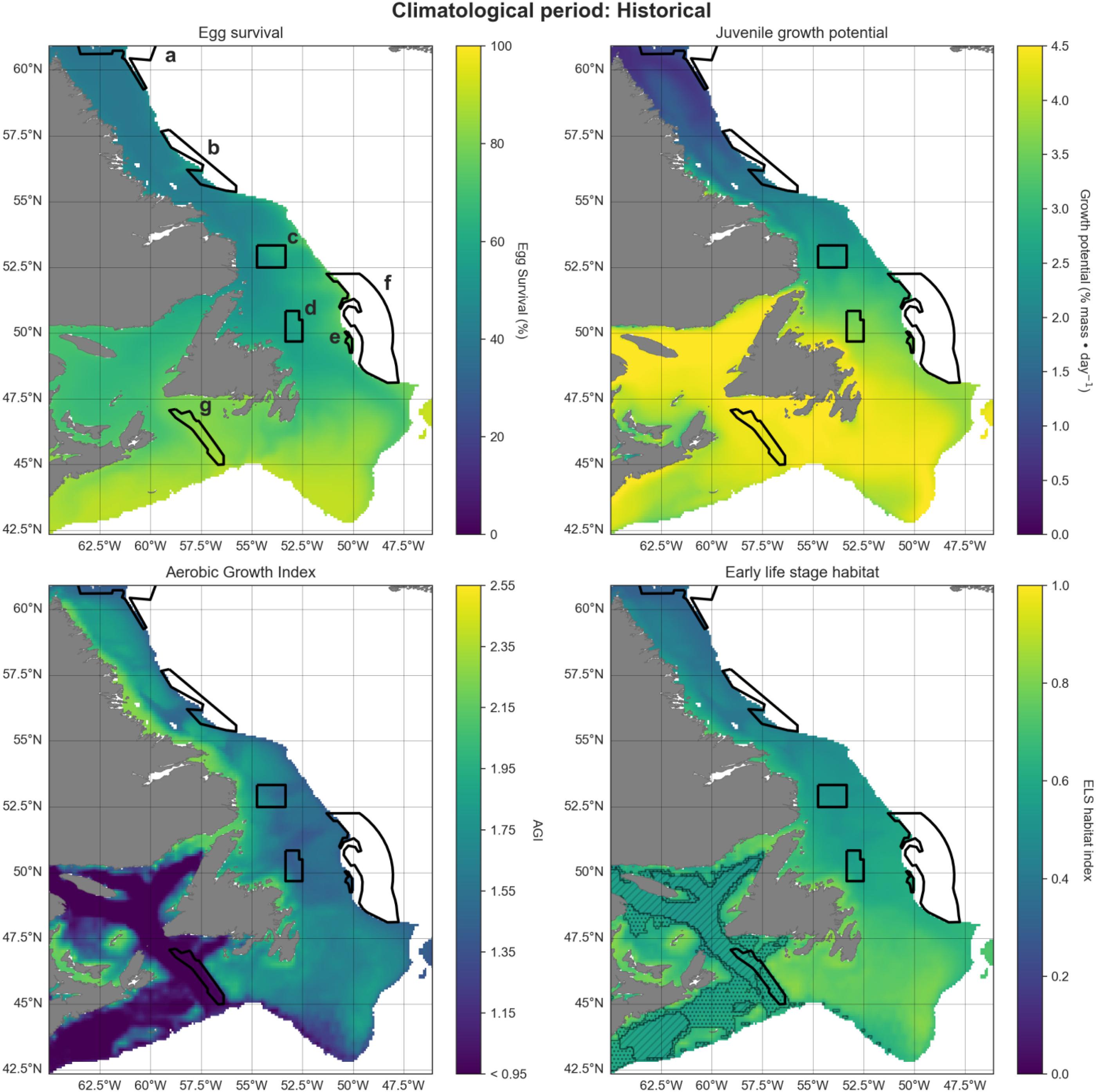
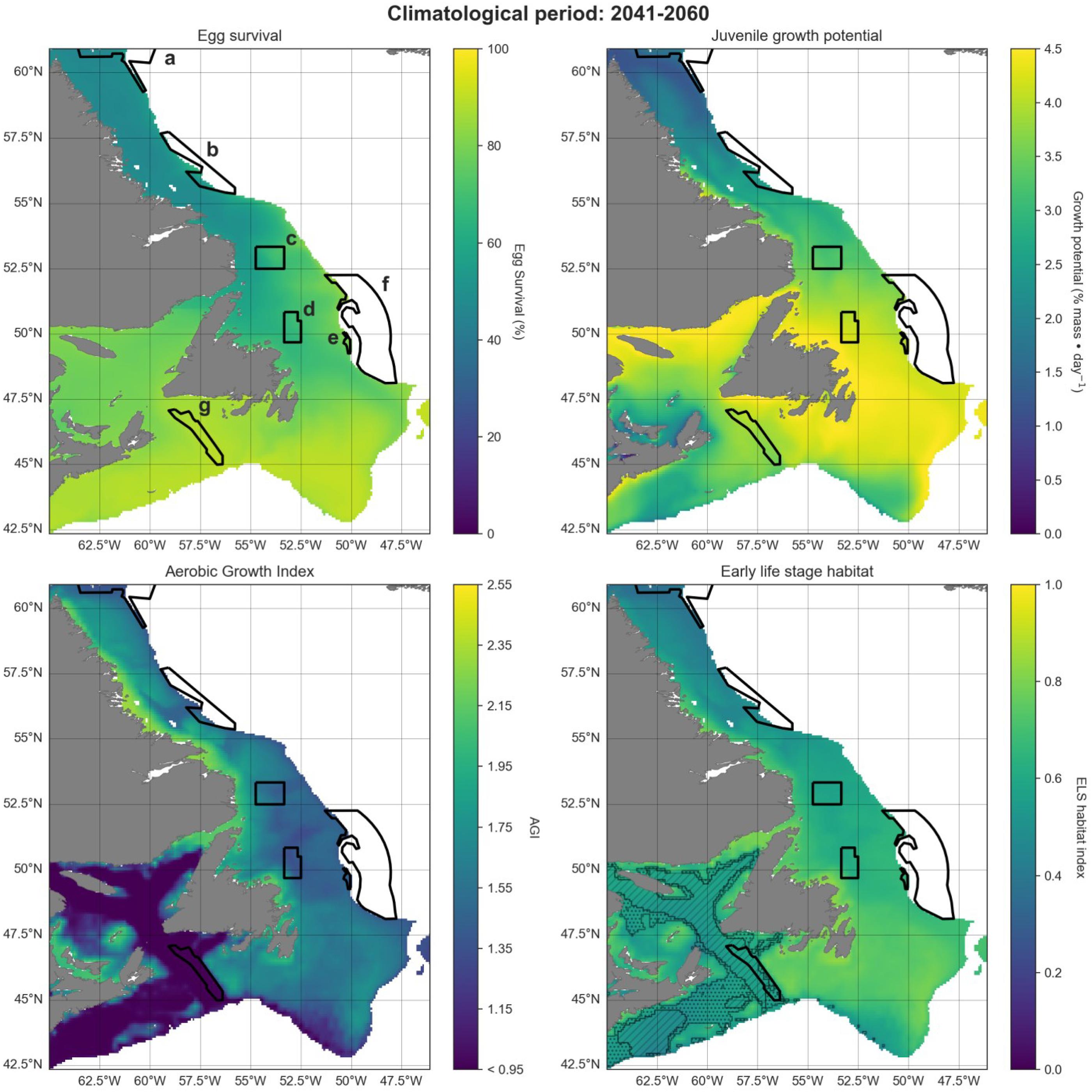
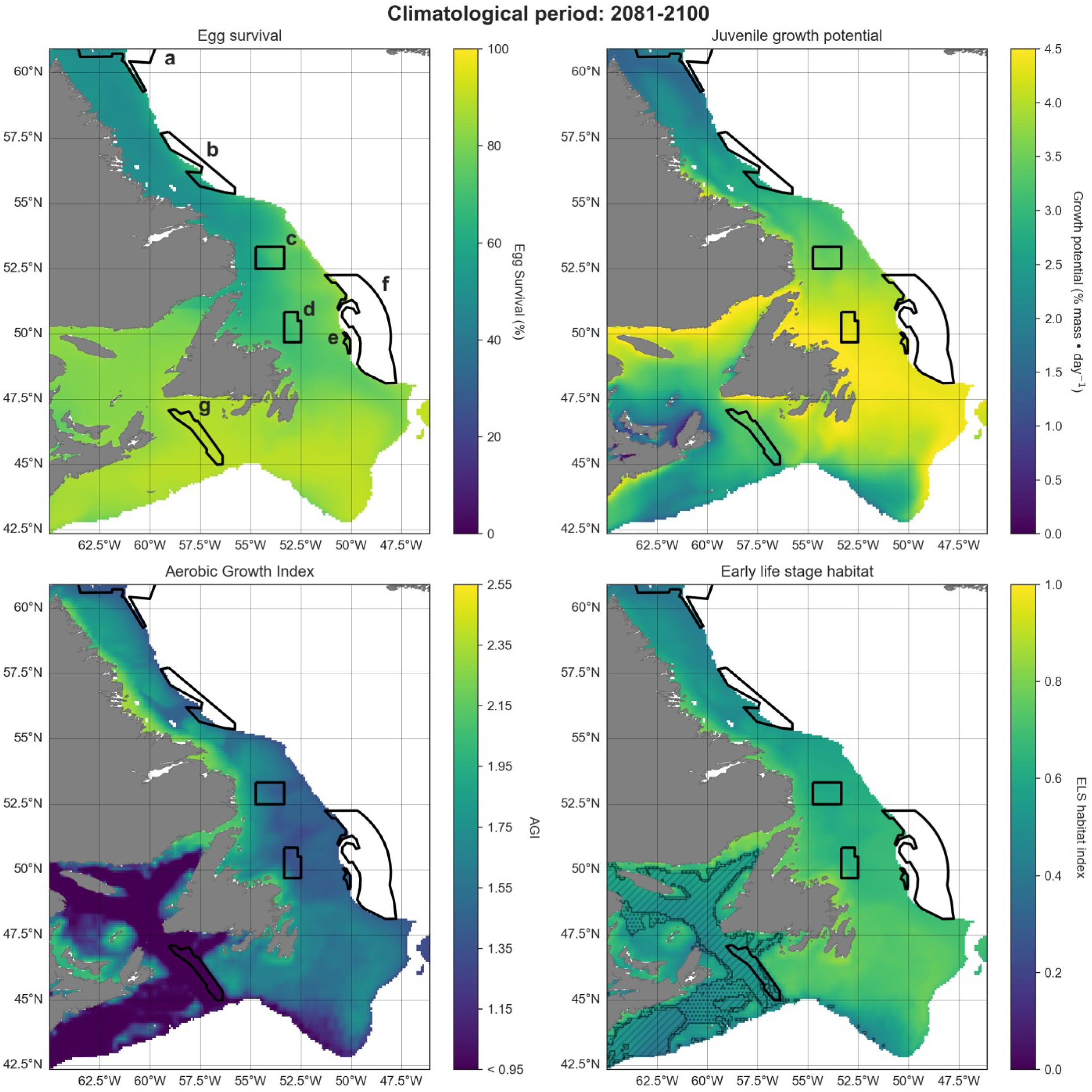
Projections were visualized in increments of 20 years, for the historical (1993–2020), mid-term (2041–2060), and long-term (2081–2100) similar to the approaches by the IPCC. Maps show the 600 m isobath and shallower regions from 42.5°N to 61°N latitude. Preferred Atlantic cod habitat in this region extends to 450 m depth although they have occasionally been observed as deep as 1000 m (Appendix A). We chose 600 m to provide a buffer to analyze conditions both deeper and more northerly from current optimum habitat, into areas where cod ranges are hypothesized to expand.
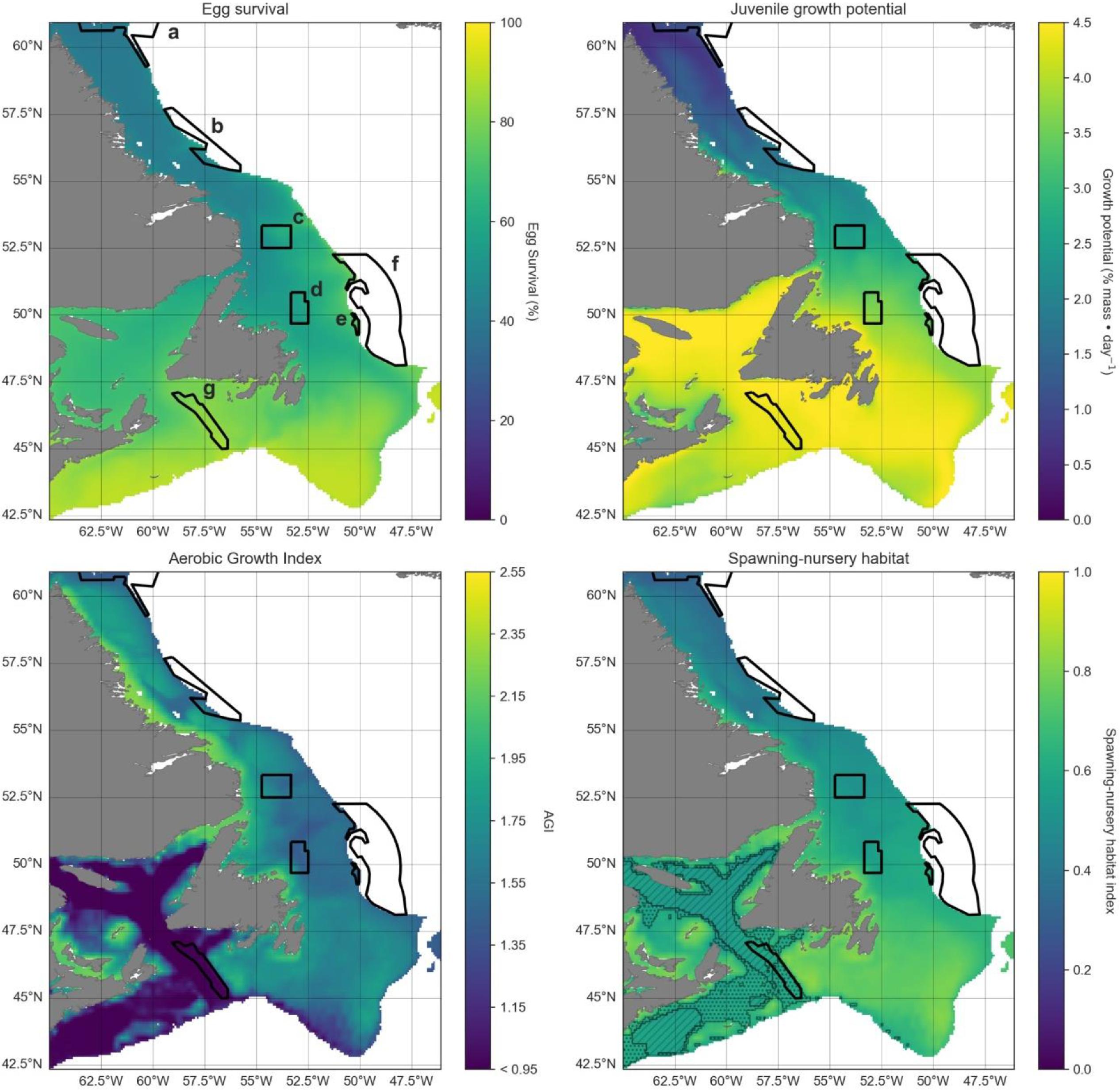
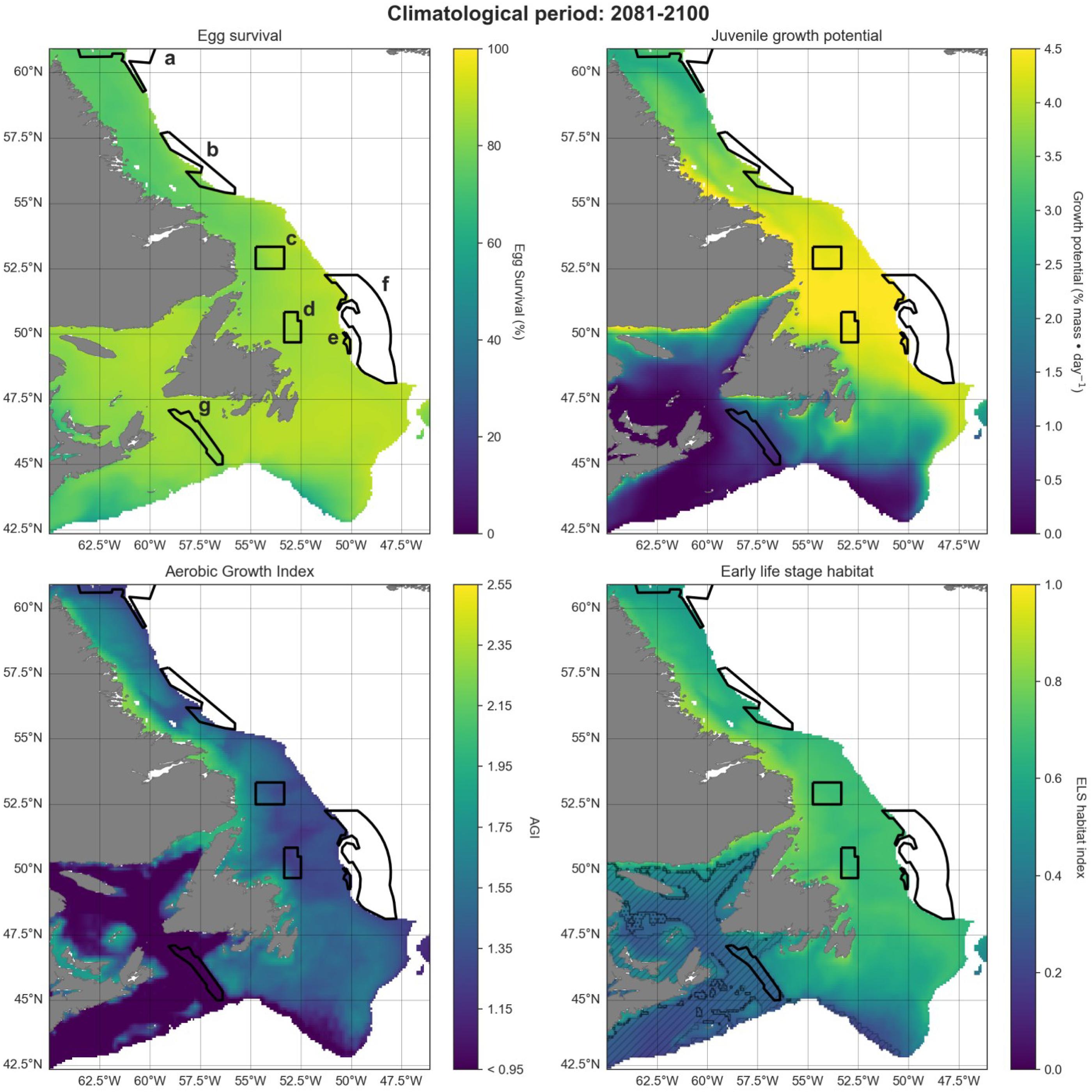
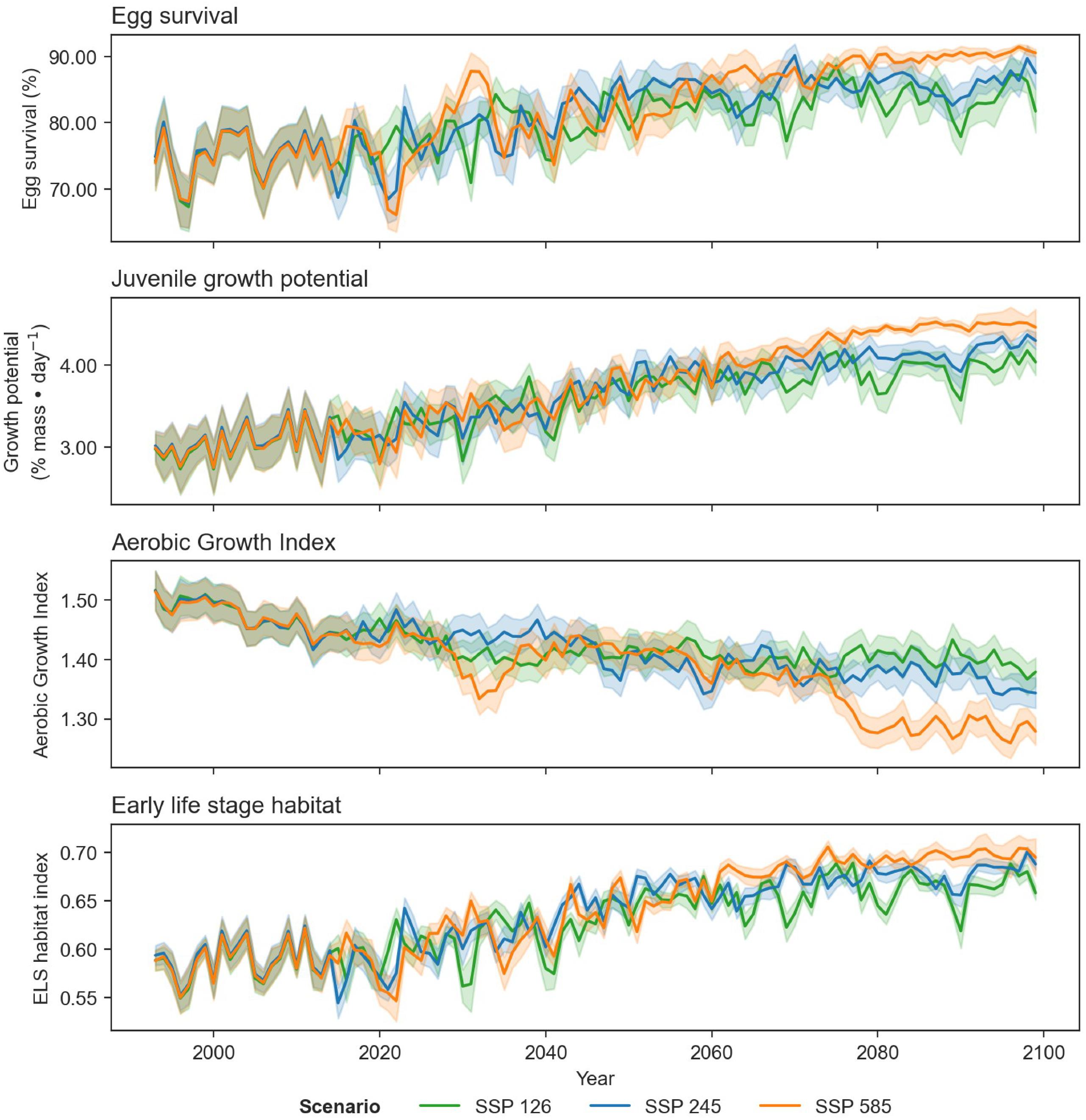
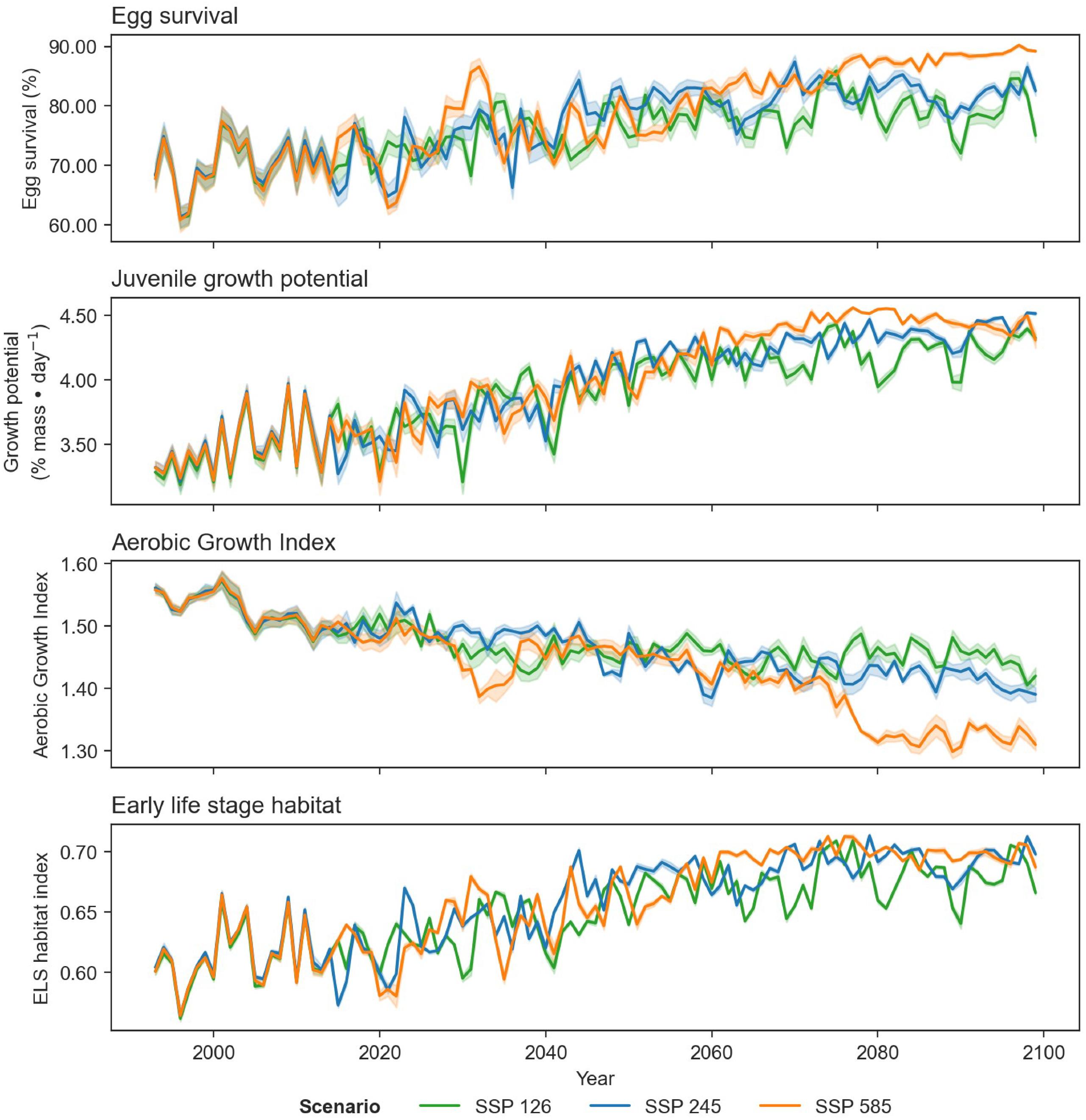
Cod habitat indices
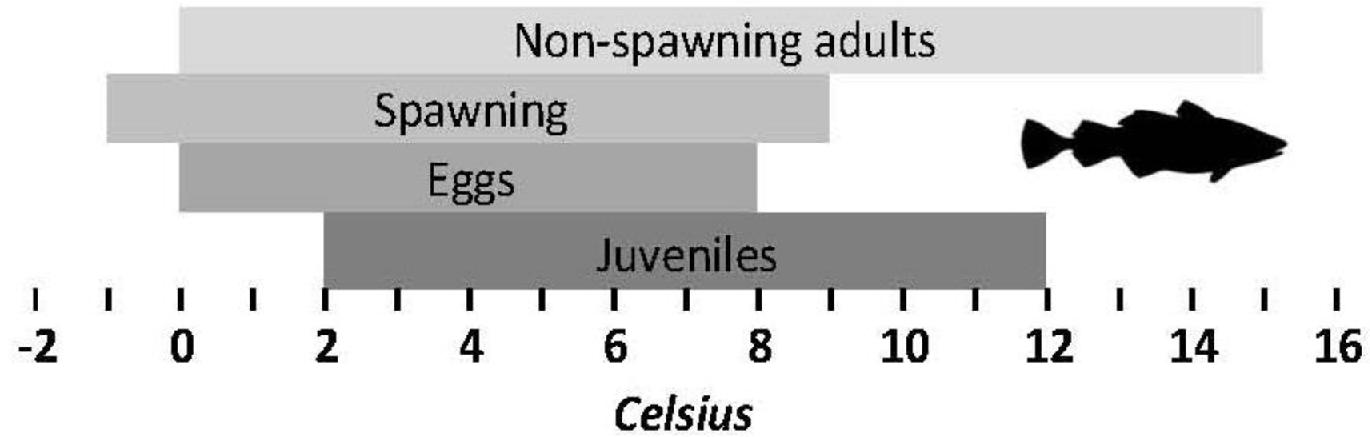
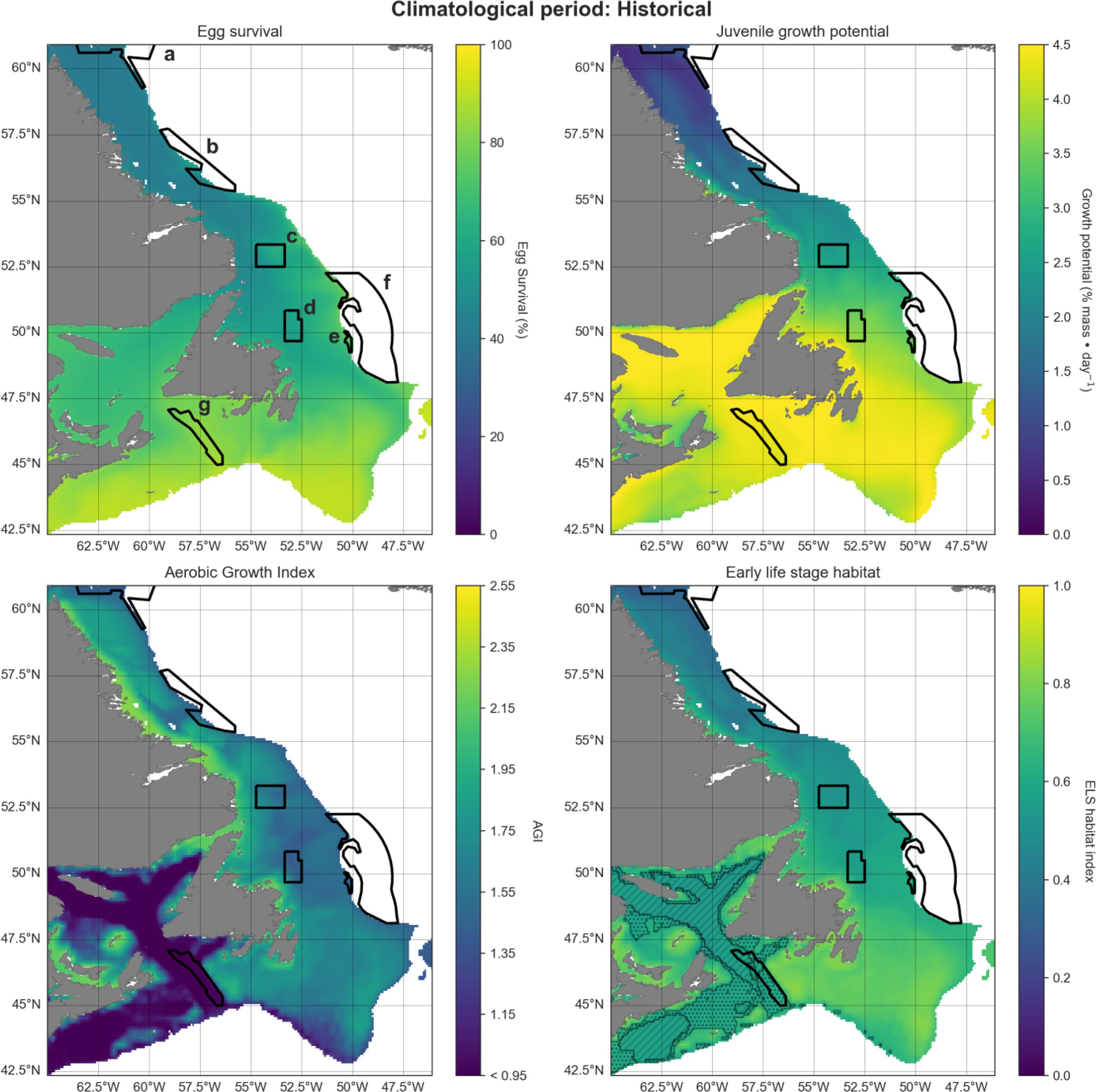
We developed a spawning-nursery habitat index that considers the needs of Atlantic cod during their most critical life stages, including eggs, juveniles, and adult female spawners. These stages are critical for recruitment and can be constrained by environmental stressors. For eggs we focus on hatching and for juveniles on growth, both of which are influenced by temperature. Habitat suitability for adult spawners was defined by temperature and oxygen. Thermal and oxygen limits of each life stage are shown in
Fig. 2. Thermal limits for adult spawners, juveniles, and eggs are relatively narrow, compared to those of adult non-spawning cod. We also employ maximum depth as a limiting factor (see above). To understand how climate change may impact potential cod spawning-nursery habitats, we quantified changes in conditions for essential life history events driven by temperature and oxygen conditions for specific periods of the year. We attempted to include an indicator linked to prey availability and recruitment (chlorophyll;
Kristiansen et al. 2011;
Lunzmann-Cooke et al. 2021) in our projections but uncertainty of this index was too large to draw substantive conclusions (
Fig. 3).
Finally, we quantify the spawning-nursery habitat index (H) by additively combining the annually averaged and normalized (0–1) three parameters into a single index. This index H represents the predicted annual spawning-nursery habitat distribution for any single 1/12-degree longitude–latitude grid cell within the study area.
Peak spawning for Atlantic cod occurs from March to May off the Newfoundland and Labrador coasts (
Scott and Scott 1988;
Hutchings and Myers 1993;
Myers et al. 1993). Pelagic egg survival was therefore calculated for these spring months using surface temperature (5 m depth) as the key driver for survival, following
Dahlke et al. (2018):
where
E is the Atlantic cod egg survival (%) as a function of ocean temperature (
T, (°C)).
Allowing for a roughly 1-month egg stage and 3-month larval stage (given cold water temperatures in this region;
Cohen et al. 1990;
McEvoy and McEvoy 1992), juvenile growth rates were calculated for the summer months (July–September) also using upper water column temperatures at 5 m depth. Growth was calculated using the following equation from (
Björnsson et al. 2007):
where
G is the daily body mass increase (%) and
T (°C) is the ocean temperature. Values were capped at a lower limit of 0.
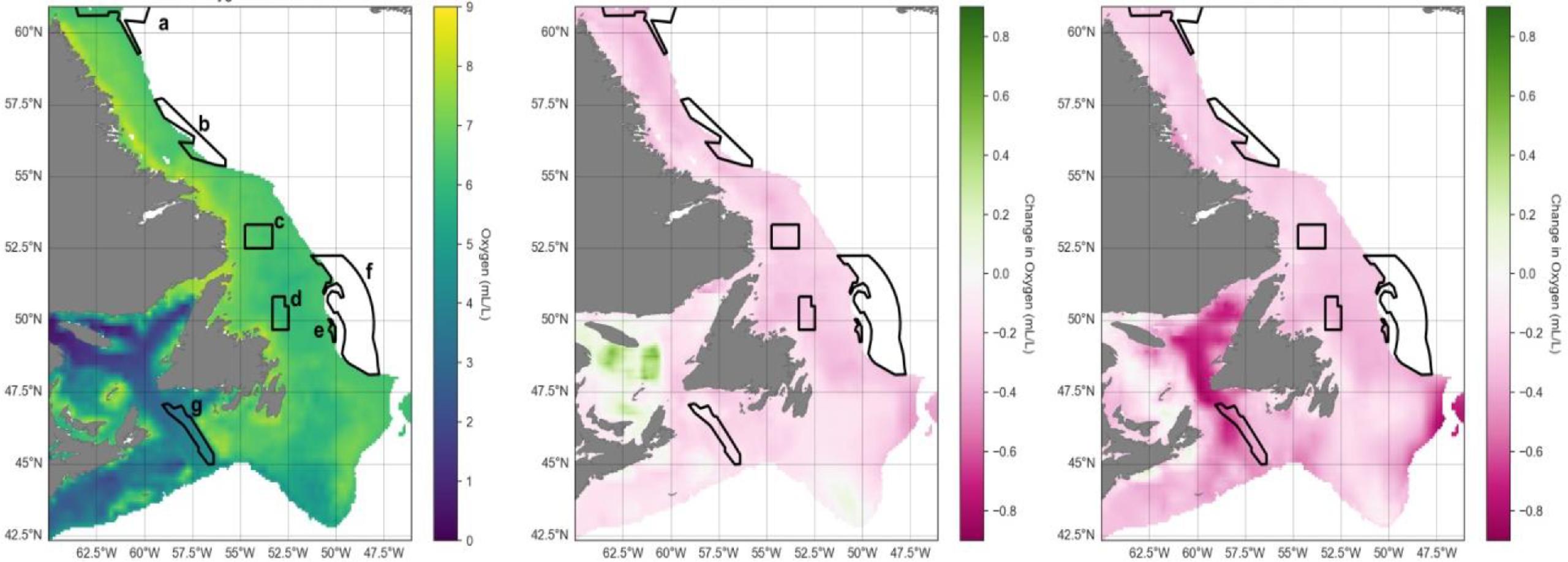
The early spring habitat of female spawners was determined using the Aerobic Growth Index (AGI;
Clarke et al. 2021). There are several benefits of using the AGI, foremost being the integrated effect of temperature and oxygen on adult metabolism and growth. We utilize Atlantic cod-specific variables to parameterize the AGI. However, the AGI makes it easy to compare temperature–oxygen parameters across species which is an important aspect beyond our cod case study for this network of closures.
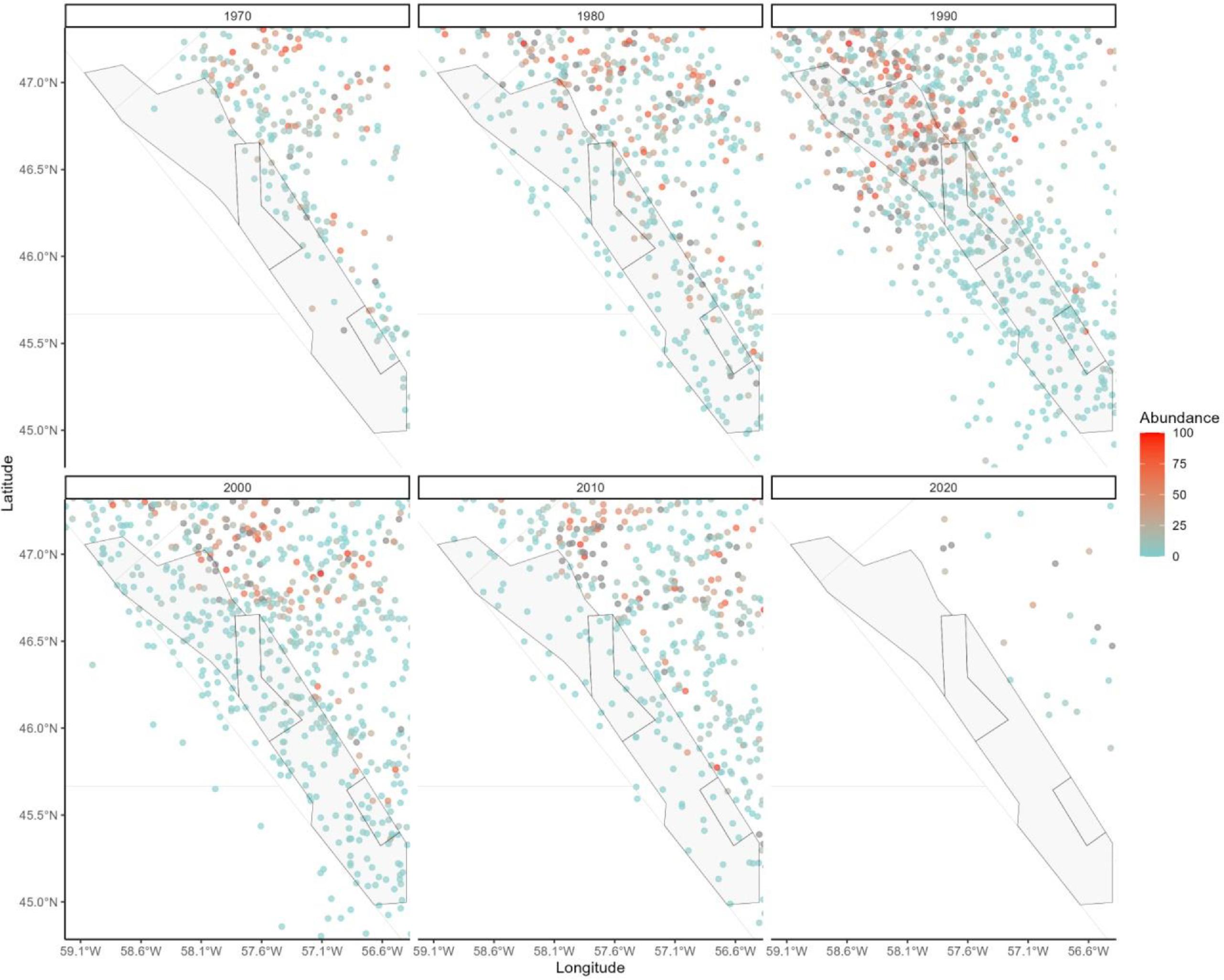
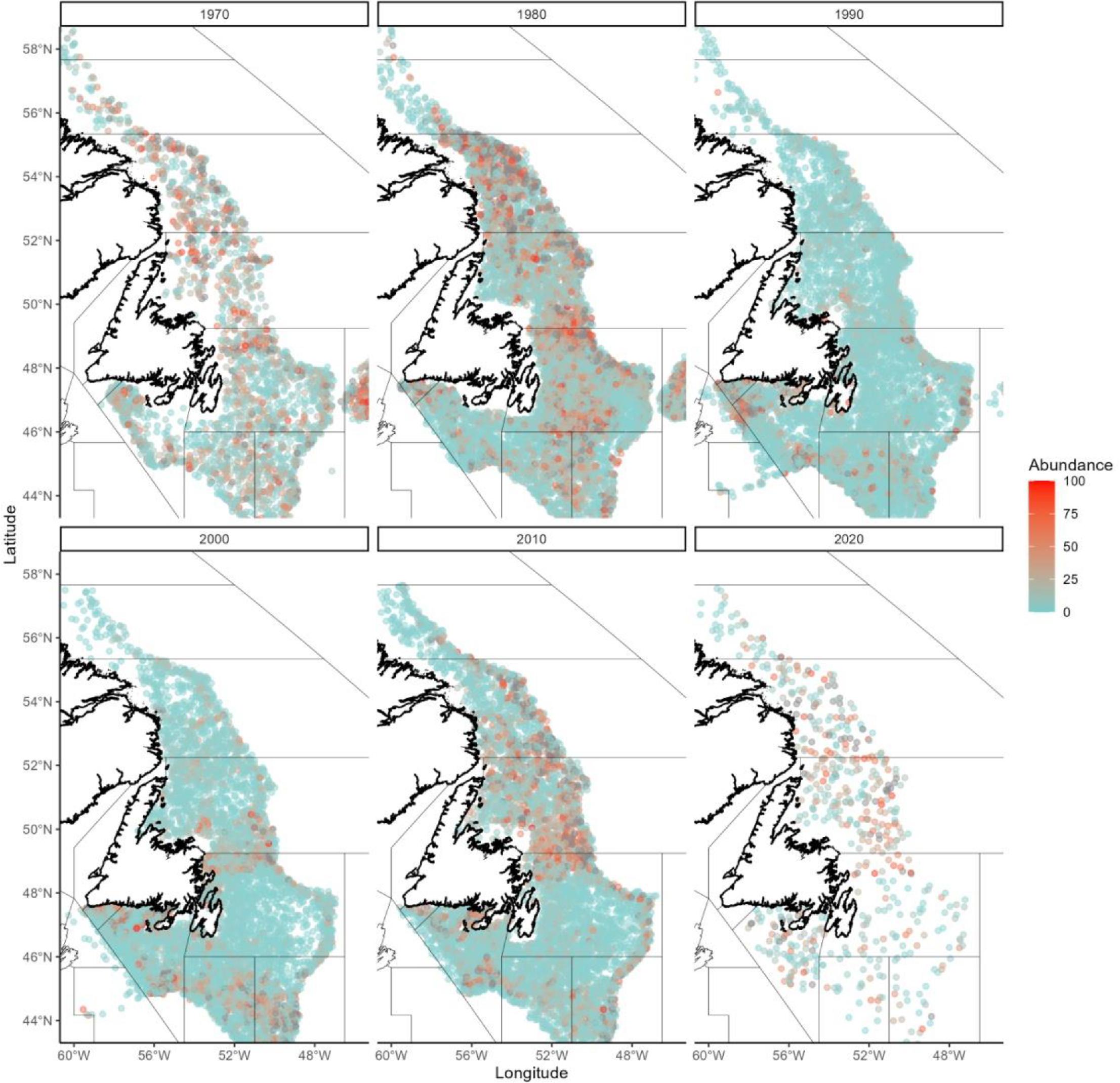
While cod may come off the bottom into the water column to spawn, adult females are mainly demersal so we used bottom temperature and oxygen levels for these calculations. We used the geographic distribution of female spawners recorded in the Ocean Biodiversity Information System (
OBIS 2023), and we correlated this with temperature and oxygen observations from the GLORYS hydrographic reanalysis to estimate the lowest AGI value where adult females are currently found, resulting in a pO
2 threshold of 0.168. Assuming this physiological threshold will remain constant, we used it to estimate future AGI based on changes in temperature and oxygen, defining the range of suitable habitats for adult female spawners. As ocean warming and hypoxic conditions are expected to increase in the future, the physiological performance of marine species will be affected by an increase in the need for oxygen to sustain metabolism (O
2demand) and a reduction in supply (O
2supply) (
Clarke et al. 2021).
Here, pO
2supply is the ambient partial pressure (in atm), and pO
2demand is the species’ demand (in atm) at a maintenance metabolic rate (
Clarke et al. 2021). The AGI effectively measures the ratio (unitless) of a species’ oxygen supply versus demands required to sustain metabolism and growth at a given temperature. A value of 1 indicates that the species can sustain essential behaviors like swimming, feeding, and predator avoidance but not growth. For more information, refer to
Clarke et al. (2021). For this analysis, we set all AGI values < 0.95 to 0.95. This threshold was implemented since female spawners are not currently observed in areas with an AGI < 0.95. We are assuming there is no spawning below AGI = 0.95.
Cote et al. (2021) modeled two cod life stages together, and we expanded on that approach. Each of our three separate indices (eggs, juveniles, and spawners) was averaged across its respective 3-month period to produce one value per year, and then normalized from 0 to 1. For the AGI, values between 0.95 and the maximum AGI value across the study area for all scenarios (2.43) were normalized to 0–1. This provided a zero baseline for comparisons between life stages and different climate scenarios.
We employed an additive modeling approach for our spawning-nursery habitat index, desiring a conservative method, and interested in benefits of closures for some cod life stages even if not suitable for all life stages. The combined annual index (
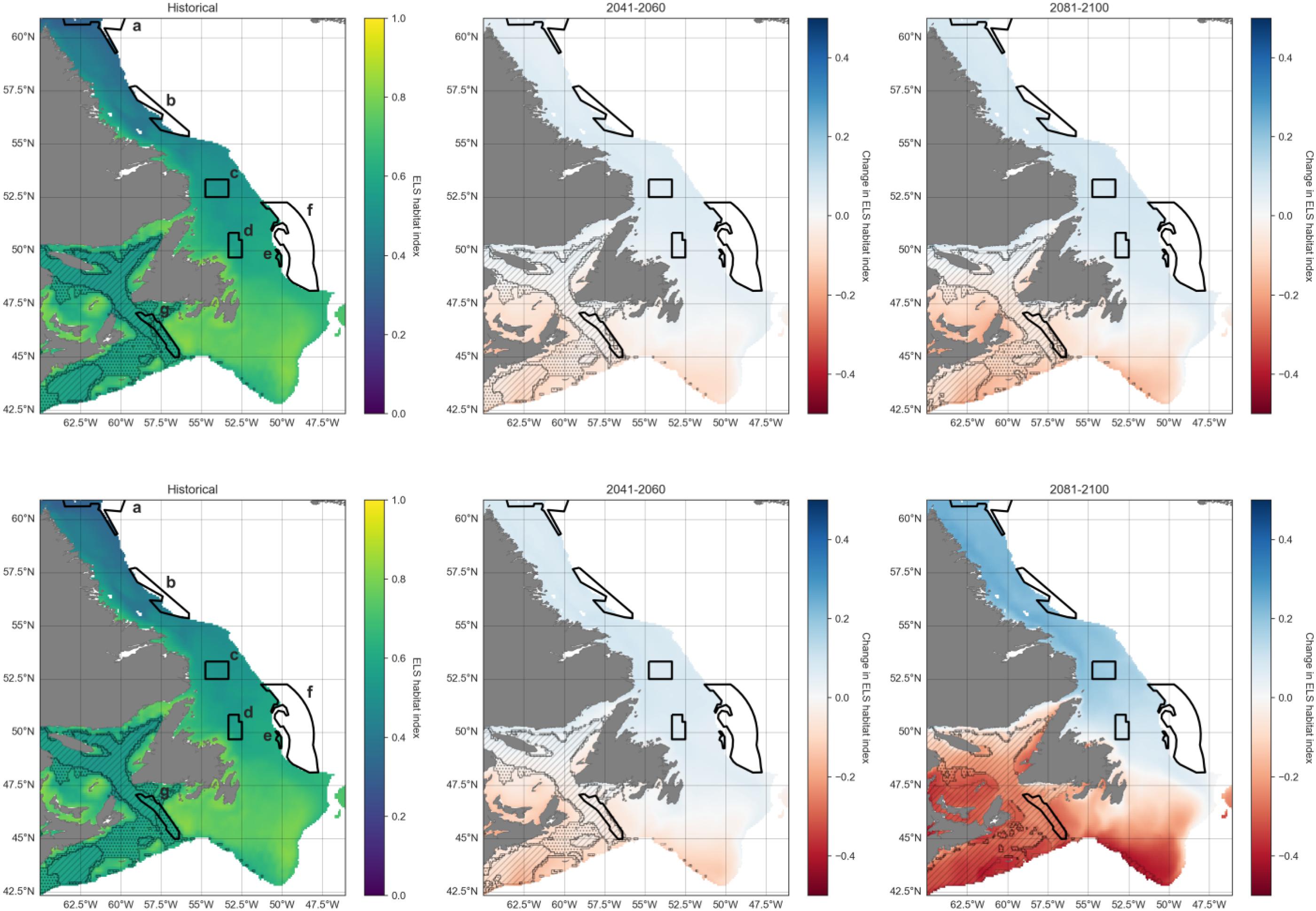
H) was obtained by averaging the normalized egg, juvenile growth, and AGI indices. One drawback of this approach is that it is difficult to identify potential bottlenecks where low habitat quality for one life stage may limit another. To visually highlight such bottlenecks, we indicated areas on the spawning-nursery habitat index maps where at least one metric was equal to 0 (post-normalization) when averaged over all years for each time period. Further, we highlighted areas where at least one metric had a value of 0 in any of the years within each time period.
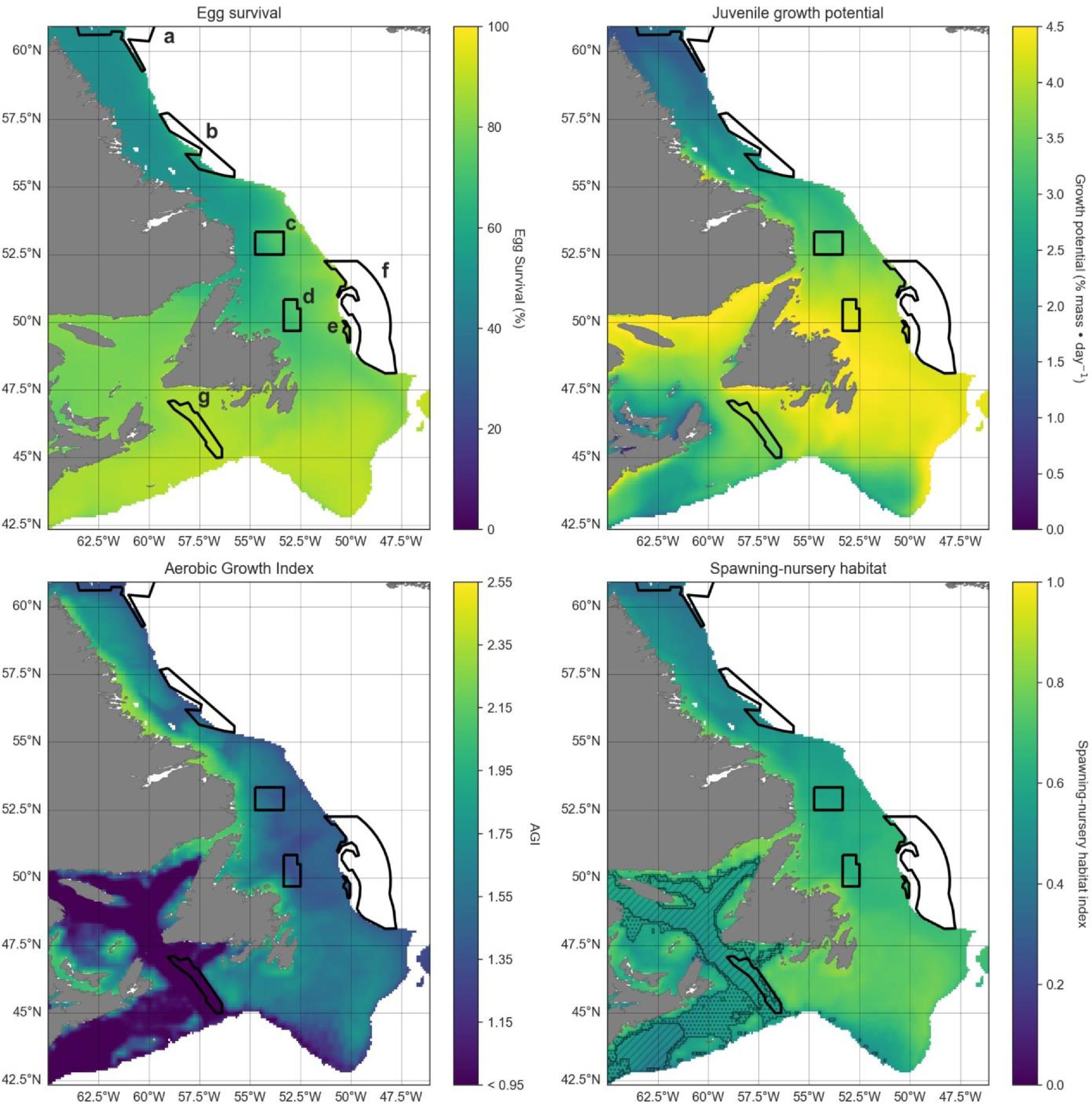
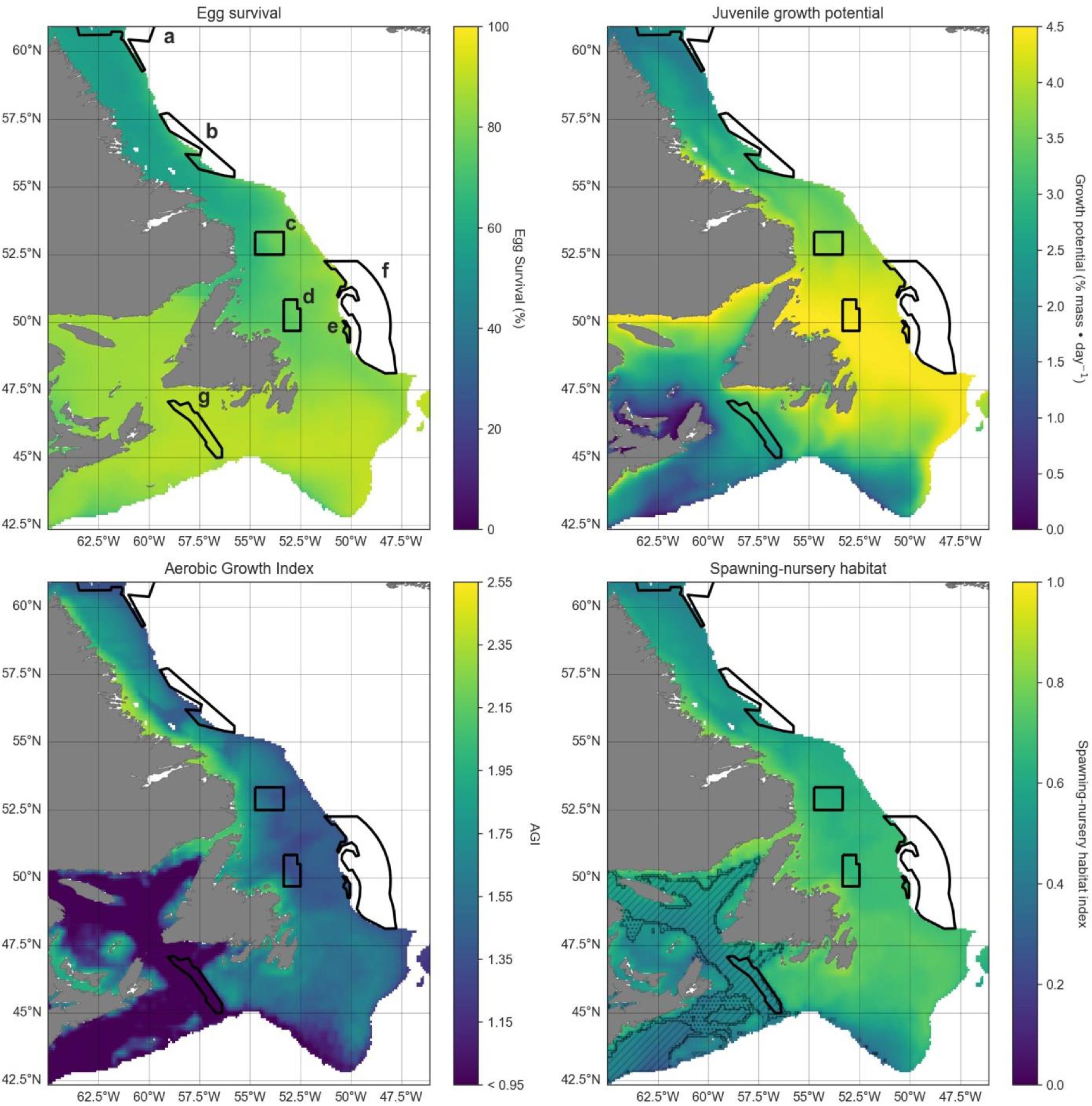
Habitat calculations were performed for the study area to map potential spawning-nursery habitat distribution over time. We did these calculations for three different climate scenarios SSP1-2.6, SSP2-4.5, and SSP5-8.5. Plots are shown for the “middle of the road” scenario SSP2-4.5, with % differences in mean spawning-nursery habitat index values between the scenarios shown in
Table 2, and other results are available in
Figs B1–
B7.
Current boundaries for MPAs/OECMs listed in
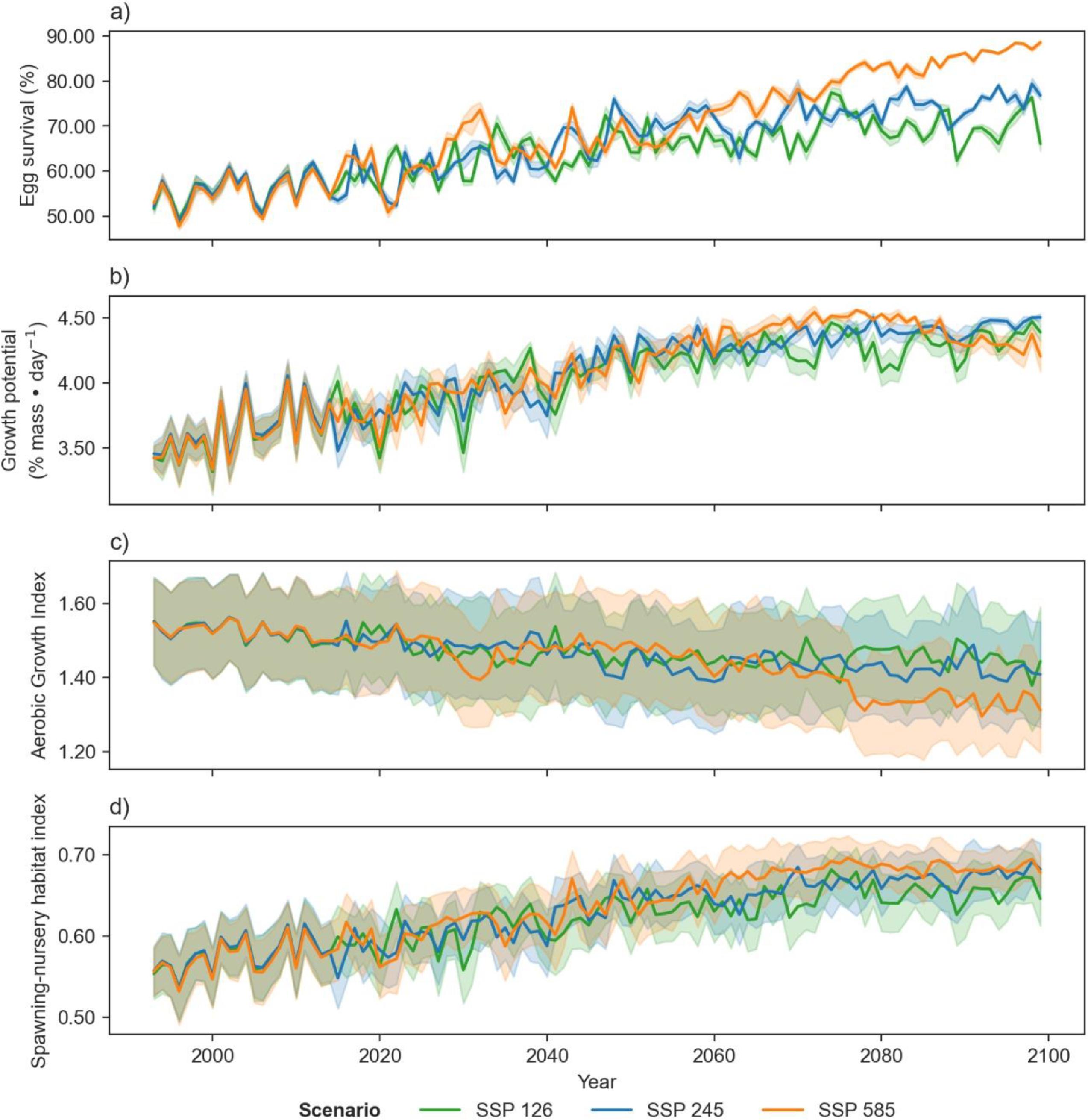
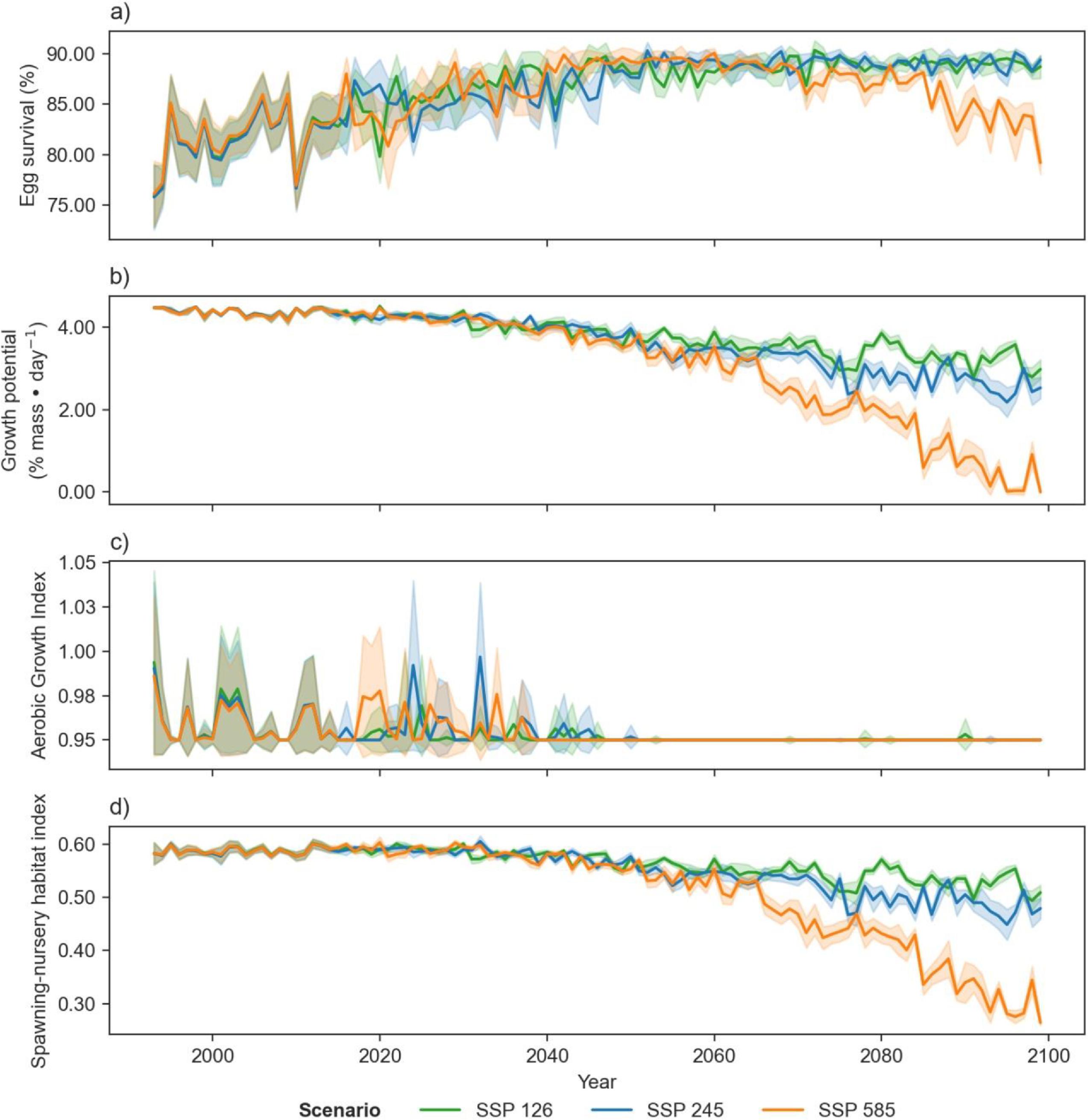
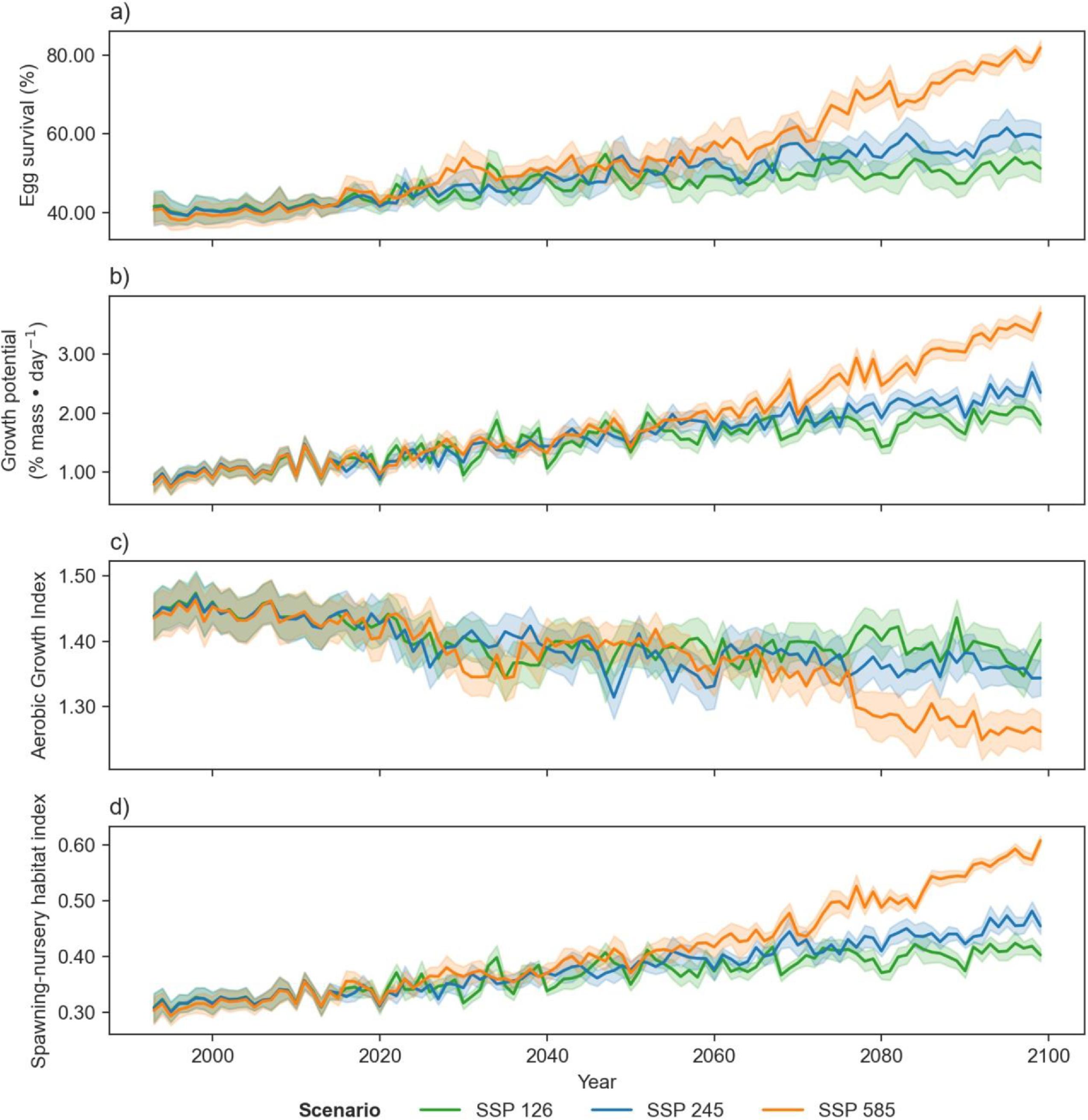
Table 1 were also mapped and time series extracted for each, to analyze index trends over time due to climate change and pinpoint potential areas of refugia or vulnerability. Funk Island Deep and Hawke Channel Closures explicitly mention cod in their conservation objectives. The Laurentian Channel MPA, Northeast Newfoundland Slope Closures, Hopedale Saddle Closure, and the Hatton Basin Conservation Area do not list cod as a specific conservation objective but confer bottom protections and occur at least partially within the preferred depth range for cod (<600 m); other MPAs and closures are deeper. For comparison, we focus here on the Funk Island Deep Closure in the center of current optimal cod habitat, as well as Laurentian Channel MPA in the south and Hatton Basin Conservation Area in the far north of the region (results for other closures are available in Appendix C).
Discussion
The effects of climate change are likely to be manifested in complex ways across species and life stages, based on local oceanography and bathymetry and ecological interactions. Climate vulnerability assessments emphasize the need to consider multiple life-history constraints in forecasting fish distributions under changing environments (e.g.,
Hare et al. 2016;
Brander 2019). However, mechanistic risk assessments targeting multiple vulnerable life stages in a climate-scenario context are rare (but see
Gamliel et al. 2020;
Cote et al. 2021). Having a habitat index which aggregates various life stages can improve ease of application to management and be helpful for visualizing overlap in hotspots and in siting new protected areas. However, a single index may obscure individual mechanisms at play. Thus, there is value in examining both individual and combined indices.
We project suitable habitat for all considered Atlantic cod life stages to shift poleward by 2100, similar to other habitat predictions (
Fulton 2011;
Hazen et al. 2012;
McHenry et al. 2019;
Cote et al. 2021). However, changes to the distribution of suitable habitat vary broadly among life stages. Our results are similar to those of
Cote et al. (2021) with suitable cod egg and juvenile habitat shifting east and north. However, with increased spatial model resolution (7 vs. 100 km) and improved resolution of nearshore processes (CMIP6 vs. CMIP5), our maps contain more fine-scale information. Higher coastal resolution is particularly important for cod as juvenile life stages are thought to rely heavily on coastal habitats (
Gregory and Anderson 1997;
Anderson and Gregory 2000). These habitats were also highlighted as highly suitable in our projections. Additionally, we examined adult spawning habitat as a function of temperature and oxygen through the calculation of the AGI, moving beyond single-factor (i.e., temperature) habitat suitability projections that may underestimate climate-related habitat fragmentation (
McHenry et al. 2019).
For other cod species (e.g., Pacific cod
Gadus macrocephalus), narrow thermal preferences of spawners and embryos collectively result in spatial and/or temporal constraints on spawning habitat (
Rijnsdorp et al. 2009;
Ciannelli et al. 2021), potentially representing a limit on their ability to adapt to changing climate (
Dahlke et al. 2020;
Ciannelli et al. 2021). Atlantic cod occupy diverse thermal habitats across their range (
Brander 2019); however, the thermal tolerance of spawners is much narrower than those of juveniles or non-spawning adults, and the tolerance of eggs narrower still (
Fig. 2;
Brander 2019). When considered alone, temperature increases in this cold-water system appear to be beneficial, improving conditions for Atlantic cod even in the south of the region. Yet, future adult spawning habitat changes will be largely driven by oxygen limitation (
Brennan et al. 2016). So while egg and juvenile habitat may remain suitable or even improve throughout most of the study region, our multi-factor approach predicts spawning habitat will decrease faster in the south due to declining oxygen levels. Exposure to hypoxia can lead to reduced food consumption by cod, lower growth rates, and decreased body condition (
Chabot and Dutil 2005;
Chabot et al. 2013;
Casini et al. 2016), leading to poorer quality fisheries catches and negative effects on spawning and recruitment. Oxygen levels are thus an important indicator to monitor in the Laurentian Channel closure and the surrounding region for Atlantic cod and other species.
We overlaid cod habitat projections with existing protected area boundaries, revealing both areas of climate refugia and threat. Projections of increasing cod egg habitat suitability were associated with all existing area closures, at least for those portions occurring in waters shallower than 600 m. The Hatton Basin Conservation Area in the north will exhibit the largest increase in suitable cod habitat in the network by 2090–2100 (
Fig. 8) with the Hawke Channel and Hopedale Saddle closures also exhibiting large increases along the Labrador shelf. None of these offshore areas are currently known as productive areas for juveniles. Instead, prevailing literature suggests that most juvenile production occurs in the nearshore zone (
Gregory and Anderson 1997;
Ings et al. 2008). But juvenile cod have recently been detected in offshore shelf environments with baited cameras (C. Morris/Fisheries and Oceans Canada, pers. comm. 2023) and may become more common as stocks rebuild from the collapse in the early 1990s. These observations suggest existing offshore closures could contribute habitat for stock recovery, particularly for settling juveniles that benefit from structurally complex habitats (
Tupper and Boutilier 1995;
Gotceitas et al. 1997;
Linehan et al. 2001;
Laurel et al. 2003;
Bradbury et al. 2008;
Gorman et al. 2009;
Renkawitz et al. 2011) that are protected from habitat disturbance.
Along the Labrador shelf between Hawke Channel and Hatton Basin, where Atlantic cod spawning-nursery habitat is expected to increase substantially over the next century, few spatial protections currently exist within suitable depth ranges. Hopedale Saddle is intermediate to these areas but most of this closure is too deep for cod (83% is >600 m;
Table 1), nor are coastal habitats protected north of Gilbert Bay, Labrador (52.6°N). New protected areas are being explored within the land claim waters of the Labrador Inuit (represented by the Nunatsiavut Government), which span a broad latitudinal gradient (∼54 to 61°N). The north–south orientation of these waters is well positioned to accommodate persistent latitudinal shifts in suitable habitat for Atlantic cod and other coastal species well into the future. Decisions on protected area implementation in this area will depend on many factors, most notably how they serve Nunatsiavut beneficiaries. However, healthy and abundant cod stocks would offer beneficiaries both increased food security and improved economic prospects.
Spatial mismatches are expected between suitable spawning habitats and those that support strong egg survival and juvenile growth. It is unclear, however, to what degree these spatial mismatches may limit populations as Atlantic cod eggs and larvae are passively transported with prevailing currents (
Bradbury et al. 2008;
Ings et al. 2008). As such, our modeling approach reflected an interest in depicting where habitat suitability may be good for some but not necessarily all cod life stages. This was particularly relevant for the southwestern part of our study area and up into the Gulf of St. Lawrence (see hatched pattern in
Fig. 6). For example, the Laurentian Channel MPA exhibits increasingly unsuitable conditions for adult spawners, but relatively good egg and juvenile growth habitat suitability for much of the coming century. Within the study area, shifts in suitable spawning habitat could have additional implications for egg/larval survival if currents advect eggs into unfavorable conditions (
Bradbury et al. 2008). However northward shifts in spawners still leave the possibility that southerly flowing currents will advect eggs/larvae to habitats further south that retain value as juvenile rearing habitat (
Davidson and DeYoung 1995;
Pepin and Helbig 1997;
Bradbury et al. 2008). Incorporating the influence of currents and wind into analyses will be important to predicting the effects of shifting spawning habitats particularly since processes, such as the Labrador Current transport and mixing on the shelf and shelf-edge front are anticipated to change with climate (
Narayanan et al. 1991;
Pepin and Jamieson 2013;
Han et al. 2019).
Projecting habitat suitability into the future is challenging and uncertainty increases the farther we predict beyond empirical data. Individual climate models within our ensemble differ significantly and our carbon emission scenarios span a wide range. Therefore, our results may be more useful for evaluating relative outcomes across a range of scenarios rather than determining absolute values of habitat suitability. Additional to the uncertainty of parameters included within our model, is the uncertainty associated with physical (e.g., salinity;
Brander 2019) and ecological (prey, predators, and competition) factors external to our model.
External physical environmental factors influencing Atlantic cod distribution and recruitment (
Brander 2019), particularly for passively drifting eggs and larvae, include salinity (
Shackell et al. 2013), currents, and wind (
Davidson and DeYoung 1995;
Ings et al. 2008). Ecological factors, such as prey availability, are important to juvenile growth and survival were also not accounted for in our model. The juvenile growth curves used to calculate the projected surfaces were fitted from experiments where cod had access to unlimited food (
Björnsson et al. 2007), whereas juvenile growth will differ when exposed real world prey fields (
Brander 2019) that can be more sparse, patchier, and potentially temporally mismatched to the juvenile growth period. Prey availability would also be expected to influence the distribution of spawners. Finally, Atlantic cod can adapt to different thermal regimes (e.g.,
Barney et al. 2017;
Sinclair-Waters et al. 2018;
Brander 2019), but it remains unclear whether adaptation can keep pace with the rapidly changing climate. Despite these uncertainties, responses of Atlantic cod to past warming events (1925 to the late 1940s) in the northwest Atlantic resulted in responses that were qualitatively similar to those presented here (i.e., pronounced northward shifts in distribution;
Brander 2019).
Forward-looking analyses such as presented here offer opportunities to proactively shape management and resource industries in a way that maximizes ecosystem and species’ sustainability and resilience in the face of climate change. While a projected increase in cod egg habitat potentially presents an ecological opportunity, spatio-temporal mismatches, shifts in optimum juvenile habitat, and decreases in adult spawning habitat may represent spatio-temporal bottlenecks. Aside from assessing the functionality of protected area networks under climate change scenarios, these analyses can help prioritize placement of new protected areas, identify the most relevant monitoring indicators to track the health of MPAs, and inform adaptive management for future protected area conservation objectives. For the harvesting industry and its regulators, these projections allow time to create processing infrastructure, negotiate access rights, and reallocate markets. Irreversible climate shifts are underway, already affecting the lives of residents of our study region (
McCarney et al. in press). While the results discussed throughout are for climate scenario SSP5-2.45, under a higher emissions scenario (SSP5-8.5) larger shifts in habitat suitability are predicted. More than ever, we must look forward to prepare and adapt to the impacts of climate change.