The between-day reliability of fasted circulating irisin concentrations: a cohort study
Abstract
Introduction
Methods and materials
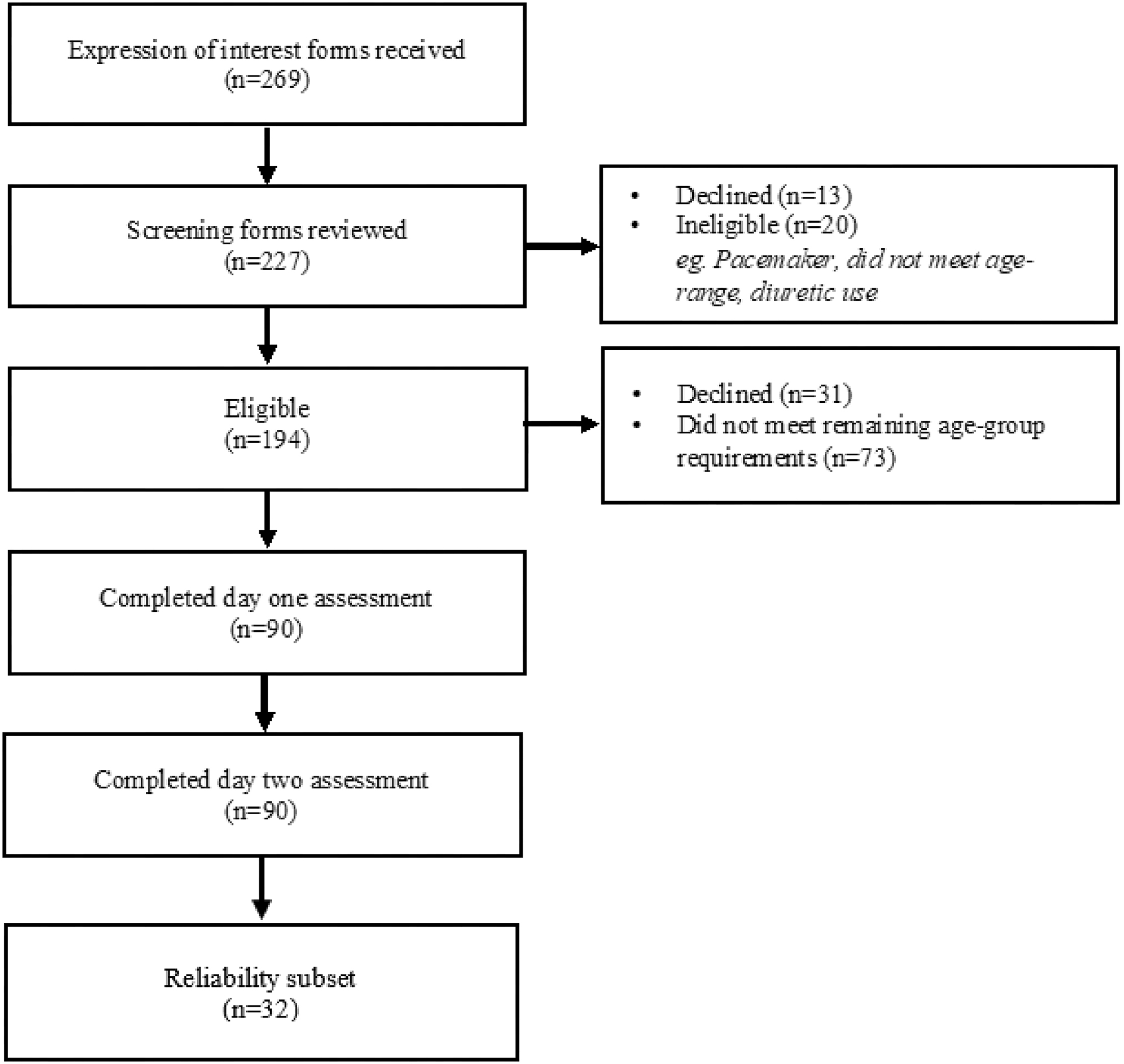
Results
| Pooled | Age-groups | ANOVA | |||
|---|---|---|---|---|---|
| All participants | Young | Middle | Older | p | |
| n | 32 | 9 | 17 | 6 | – |
| % female | 53 | 67 | 53 | 34 | – |
| Age (years) | 47.8 ± 18.2 | 23.1 ± 3.4 | 52.4 ± 6.9 | 71.7 ± 4.7 | – |
| Body mass (kg) | 75.5 ± 15.7 | 74.3 ± 12.9 | 76.2 ± 18.3 | 74.9 ± 13.9 | 0.955 |
| Height (cm) | 171.0 ± 8.1 | 173.2 ± 9.3 | 170.3 ± 7.9 | 169.6 ± 6.4 | 0.627 |
| BMI (kg.m−2) | 25.8 ± 5.0 | 24.7 ± 3.2 | 26.3 ± 6.1 | 26.0 ± 4.2 | 0.750 |
| FFM (kg) | 47.6 ± 10.2 | 48.4 ± 11.3 | 47.1 ± 10.5 | 47.8 ± 9.3 | 0.958 |
| FM (kg) | 27.3 ± 10.9 | 25.3 ± 10.3 | 28.5 ± 12.4 | 26.7 ± 7.6 | 0.776 |
| BF% | 35.9 ± 9.2 | 34.1 ± 11.4 | 36.8 ± 9.2 | 35.8 ± 5.9 | 0.787 |
| Irisin (ng.mL−1) | 0.92 (0.96) | 0.86 (2.01) | 1.04 (1.06) | 0.62 (0.93) | 0.814 |
Note: Descriptive characteristics of pooled and age-group separated participant characteristics derived from the 4-compartment model, presented as mean ± SD, irisin presented as median and interquartile range. *Significant mean difference (p ≤ 0.05) via one-way ANOVA (parametric data) or Kruskal–Wallis (non-parametric data), FFM, fat-free mass, FM, fat mass, ng.mL−1: nanograms per millilitre.
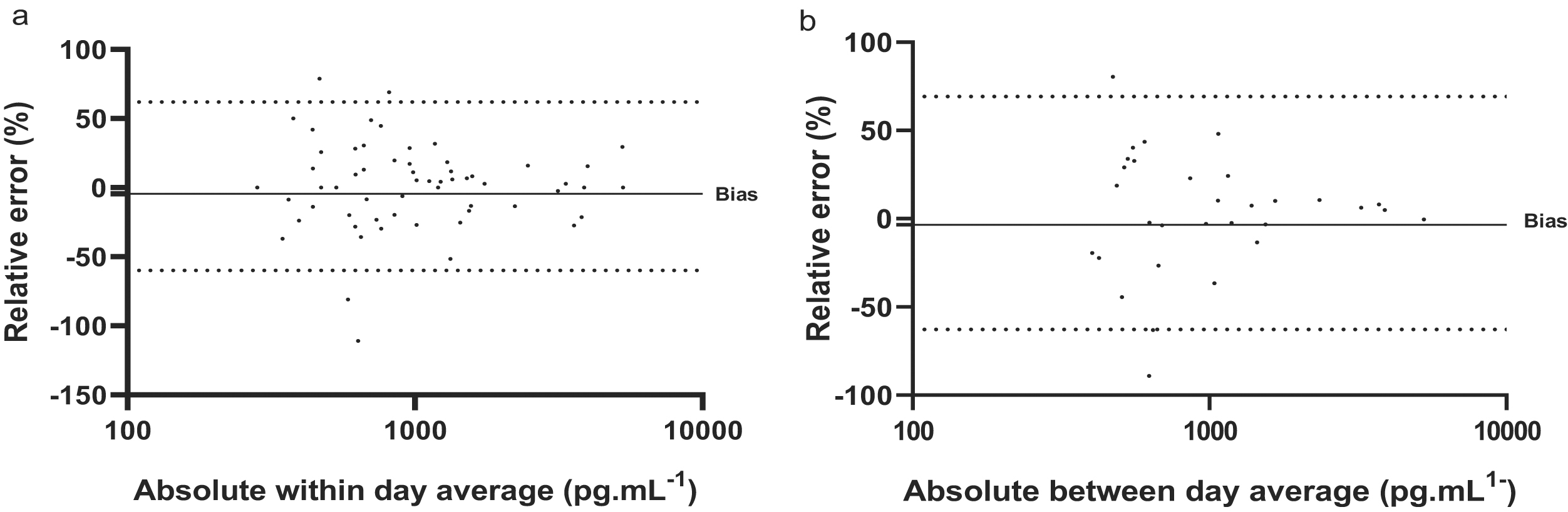
| Error type | Within-day | Between-day | |
|---|---|---|---|
| n | 32 | 32 | |
| Average valuesa (ng.mL−2) | Median | 0.93 | 0.92 |
| IQR | 1.02 | 0.96 | |
| Mean Δb | % of raw | 71.1 | 67.5 |
| Absolute | 0.66 | 0.62 | |
| 95% CIc | −1.07, 0.11 | −0.40, 1.35 | |
| TEEd | CV | 11.6 ± 10.9 | 12.7 ± 11.3 |
| ICCe | r | 0.927 | 0.963 |
| Proportional Bias | r | 0.133 | 0.221 |
| p | 0.302 | 0.201 | |
| Sexf | r | – | 0.012 |
| p | – | 0.948 | |
| Age | r | – | 0.053 |
| p | – | 0.795 | |
| Body composition | r | – | ≤0.158 |
| p | – | ≥0.875 | |
Discussion
References
Supplementary material
- Download
- 16.14 KB
- Download
- 26.81 KB
Information & Authors
Information
Published In

History
Copyright
Data Availability Statement
Key Words
Sections
Subjects
Plain Language Summary
Authors
Author Contributions
Competing Interests
Funding Information
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
There are no citations for this item
