Introduction
The main anthropogenic mode of entry for mercury into aquatic ecosystems is through atmospheric deposition of inorganic forms following emissions released from gold mining (
UN Environment 2019), or coal combustion (
UN Environment 2019), and transport via global air circulation patterns (
Pacyna et al. 2006). Atmospheric mercury is either directly deposited into waterbodies or deposited on land where it can be transported to waterways through surface runoff (
Schwesig and Matzner 2001). Following entry into waterbodies, most inorganic mercury does not remain in the water column and is subject to further transport by adsorption to particles (e.g., organic matter, minerals, microorganisms) (
Gerbig et al. 2011), which passively accumulate in the sediment. The sediment acts as a sink for inorganic mercury because of its high affinity to particles and dissolved organic matter (
Hintelmann et al. 1995). When inorganic mercury reaches the sediment, it can be methylated by bacteria under anoxic conditions (
Jensen and Jernelov 1969), primarily by sulfate-reducing bacteria that expel MeHg—likely for cellular detoxification (
Compeau and Bartha 1985). Once methylated, mercury's most bioavailable and toxic form—MeHg—can diffuse out of the sediment into the water column and be taken up by primary producers and transferred across trophic levels (
Cherry and Guthrie 1979).
Several studies indicate that selenium (Se) has a mitigating effect on the toxicity of both inorganic and organic mercury (
Parizek and Ostadalova 1967;
Naganuma et al. 1984;
Sormo et al. 2011) but the mechanism by which this occurs requires further research. Se is a trace element and essential micronutrient that animals use to form selenoproteins, which are required for key biological functions including the prevention of oxidative damage (
Beck 1999), DNA production (
Allan et al. 1999), thyroid gland function (
Vanderpas et al. 1990), and various other functions (
Rayman 2000); however, Se is toxic in excessive amounts (
Smith et al. 1937). While there have been numerous studies exploring the relationship between mercury (both organic and inorganic) and Se, and the potential for Se to protect against mercury toxicity, most of these experiments employed soluble forms of inorganic mercury (Hg
II+) and Se (e.g.,
Parizek and Ostadalova 1967;
Paulsson and Lundbergh 1989;
Moreno et al. 2014;
Cabezas-Sanchez et al. 2019;
Shang et al. 2022). This was done either directly (e.g., injection of solutions, loading of Hg
II and Se to water) (
Parizek and Ostadalova 1967;
Cabezas-Sanchez et al. 2019;
Shang et al. 2022), or indirectly (i.e., along a dissolved elemental ratio gradient) (
Paulsson and Lundbergh 1989;
Moreno et al. 2014). Few studies explore whether other pathways of exposure (e.g., through food) might affect the protective function of Se against MeHg across trophic levels (
Turner and Swick 1983;
Ralston et al. 2006;
Bjerregaard et al. 2018). This is relevant because the route of exposure through water is not as significant to living organisms when compared to dietary intake of bioavailable elements such as Se (
Franz et al. 2013) and mercury (as MeHg) (
Phillips and Buhler 1978).
Knowledge about the protective effects of Se against MeHg toxicity across trophic levels is still lacking. Existing studies examine whole ecosystems and focus on organisms at higher trophic levels rather than looking at the base of the food chain (
Gui et al. 2014;
Ouedraogo et al. 2015;
Griboff et al. 2018;
Liu et al. 2019). Current studies on Se and MeHg in lower trophic levels also focus on the accumulation of MeHg among individual organisms or identifying lethal concentration values (
Turner and Rudd 1983;
Belzile et al., 2006;
Jensen et al. 2007;
Shang et al. 2022). To our knowledge, no study has addressed the impacts of MeHg in the presence of Se on the population dynamics of an invertebrate, looking at population growth rate or maximum density as endpoints.
To address these knowledge gaps, we performed laboratory experiments on a ubiquitous species of freshwater algae,
Auxenochlorella pyrenoidosa, and a detritivore,
Aeolosoma variegatum. We hypothesized that MeHg would have a toxic effect on the population growth rate and maximum density of
Auxenochlorella, but the presence of Se would mitigate this effect. The protective effect of Se on MeHg's toxic effect in
Chlorella was demonstrated by earlier research (
Moreno et al. 2014). However, evidence indicates that
Chlorella may be able to detoxify MeHg by increasing metal-chelating compounds like phytochelatins (
Gomez-Jacinto et al. 2015), and this ability may result in negligible impacts on the population growth rate and maximum density of
Auxenochlorella in our study. We further hypothesized that exogenous MeHg would negatively impact the population density and growth rate of
Aeolosoma, but that dietary Se from
Auxenochlorella would confer protection against this toxic impact. A significant negative impact from exogenous MeHg exposure would be consistent with studies that show MeHg is toxic to various aquatic invertebrates (
Reish et al. 1976;
Best et al. 1981). Se can be toxic in excessive amounts to aquatic invertebrates (
Ingersoll et al. 1990;
Malchow et al. 1995;
Conley et al. 2009), but there is evidence in the literature to support reduced toxicity of organic and inorganic mercury in the presence of Se in aquatic invertebrates (
Nuutinen and Kukkonen 1998; Belzile et al., 2006). Lastly, we expected that dietary MeHg would decrease population growth rate and maximum density of
Aeolosoma, but this effect would be mitigated when Se was co-introduced with MeHg in the diet through
Auxenochlorella. The toxic effect of MeHg on aquatic invertebrates noted in the literature supports the expected negative impact of MeHg (
Reish et al. 1976;
Best et al. 1981), as well as Se's apparent mitigating effect (
Ralston et al. 2006;
Bjerregaard et al. 2018).
Materials and methods
To gain a better understanding of whether Se confers protection against MeHg across trophic levels at the base of the food chain, we conducted three experiments on
Auxenochlorella pyrenoidosa and
Aeolosoma variegatum.
Auxenochlorella pyrenoidosa was chosen because it is a ubiquitous species of algae that is found in freshwater lakes throughout the world (
Hodac et al. 2016). This makes it relevant for a study on environmental exposure to MeHg.
Auxenochlorella and Chlorella genera are primary producers and a food source for many freshwater species at the base of the food chain such as worms, zooplankton, bacteria, and benthic invertebrates that consume decaying algae deposited in sediments (
Singer 1977).
Aeolosoma variegatum is a small, aquatic annelid that resides in freshwater bodies across North America (
Singer 1977). This worm was chosen as it is an abundant detritovore with a relatively short lifespan of 66 ± 10 days (
Falconi et al. 2015), which allowed for convenient experimentation.
Aeolosoma reproduce asexually through paratomic fission (
Bonomi and Erseus 1984), although they are capable of sexual reproduction when growing conditions are unfavourable (
Marotta et al. 2003). Asexual reproduction is important for these experiments as it reduces genetic variability, thereby reducing the potential for genotypic differences to obscure responses to stressors across samples. As reproduction is relatively quick (rate of 1 per day depending on the life stage,
Falconi et al. 2015) this allowed for reproduction to be easily monitored over 12-day experiments.
Aeolosoma are suction-feeding detritovores and feed on decaying plant matter (
Singer 1978). Dead
Auxenochlorella should be a fitting food source for this worm to sustain itself for the 12-day duration of the experiments (
Singer 1977).
Auxenochlorella culturing
To begin the experiments, we cultured a stock of
Auxenochlorella pyrenoidosa bought from Ward's Science (New York, USA). We did this using the US EPA Method 1003.0 on maintaining green algal stock cultures (
US EPA 2002). Stock solutions for each of the necessary components were prepared by fellow graduate student Kyle Rodger (Supplementary Materials, Table S1). The medium was made by combining 2 mL of each of the stock solutions in a 2 L volumetric flask. Once the solutions were added to the flask, deionized water was added until it was topped off to 2 L. The pH of the culture medium was tested to ensure it was around 7.5, and pH was adjusted with a 0.1 M NaOH or HCl solution, as necessary. A sufficient amount (1.6 litres) of the culture medium was then filtered through a prewashed 0.45 µm pore, 47 mm diameter, membrane at a vacuum pressure of no more than 380 mmHg. The final concentrations of micronutrients and macronutrients in the
Auxenochlorella medium are shown in the Supplementary Materials—Tables S2 and S3.
Following filtration, the culture medium was placed into an Erlenmeyer flask, covered with foil and autoclaved for 30 minutes. The culture medium was left covered and allowed to sit for 24 hours, after which 3 mL from a Auxenochlorella stock culture was inoculated into the sterile media. The Erlenmeyer flask filled with the culture medium was then left at 25 ± 1° C under continuous fluorescent lighting at an intensity of 4300 lx. To promote growth, the flask was placed on a shaker that ran continuously at a speed of 100 rpm. After 12 days, the Auxenochlorella was sub-cultured and transferred into fresh media to keep the stock healthy and actively growing.
Experiment 1: Effects of MeHg exposure in Auxenochlorella across varying Se levels
Six treatments were tested for experiment 1 (
Table 1). The low level of Se selected reflects the 30-day average water quality guideline for protection of aquatic life by the Ministry of Environment for British Columbia, Canada (
BC Ministry of Environment 2014). The high treatment level of Se was deemed appropriate as it reflects the average concentration of 81 water samples (6 µg L
−1) taken along the Solomon River Basin by
May et al. (2008), which were considered high, but not contaminated. The moderate MeHg exposure level reflects the maximum contaminant level of Hg that is allowed in water (
Water Quality Association 2005).
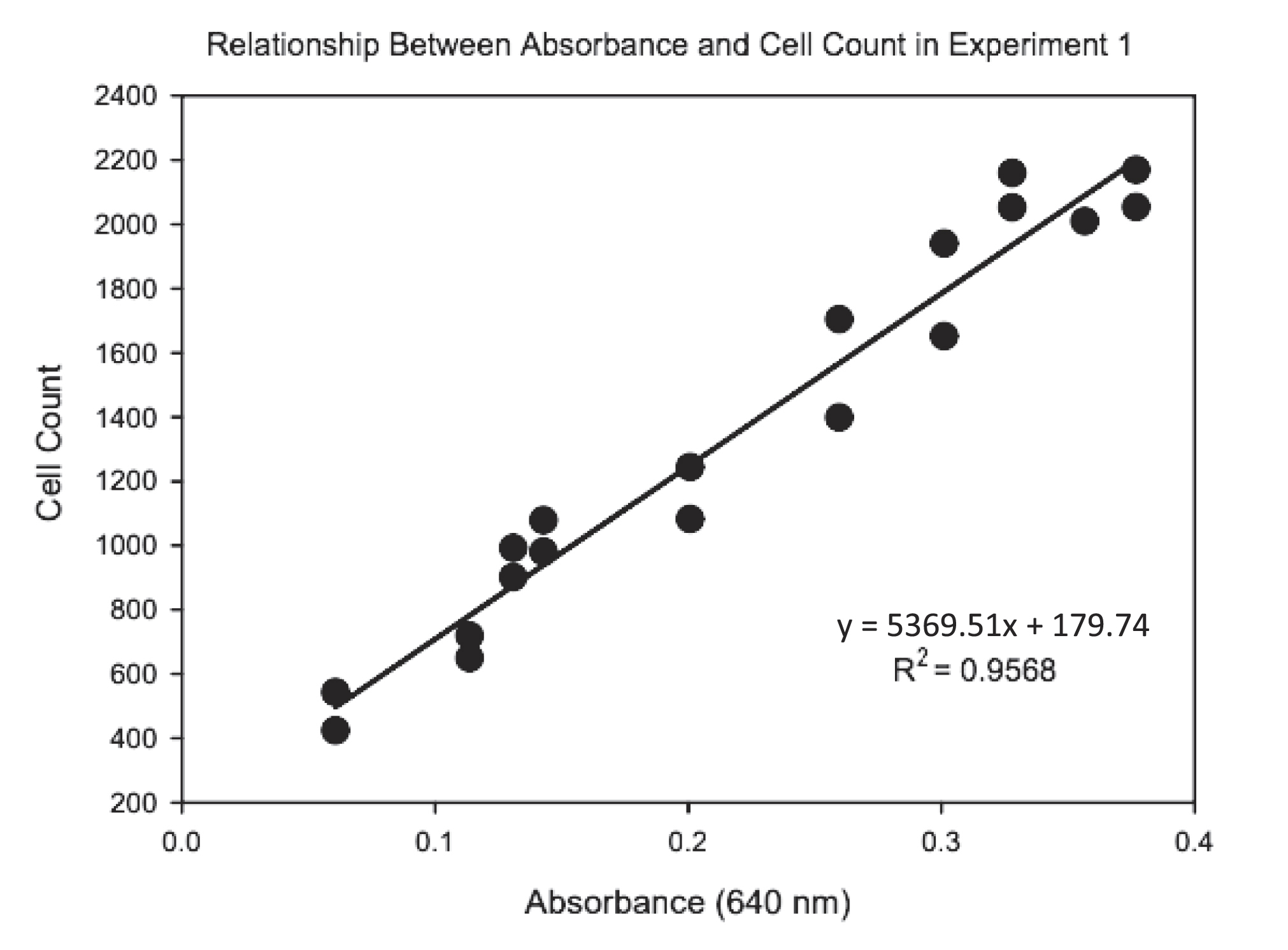
Treatments were carried out in 250 mL sterilized Erlenmeyer flasks containing 200 mL of sterilized Auxenochlorella culture medium. There were four replicates of each treatment. After the medium was ready, 2 mL of an actively growing algae stock culture was inoculated into it. Methylmercury chloride (CH3HgCl) and sodium selenate (Na2SeO4) salts were added to deionized water separately to make stock solutions used to achieve target concentrations of MeHg and Se for each treatment. The desired amount of MeHg and Se were pipetted into each vessel, and they were left to grow for 21 days. The flasks were manually shaken every day to provide gas exchange and promote growth. The growth of each treatment was monitored every other day by recording the % transmittance value using a spectrophotometer set at 640 nm. The transmittance values were then converted into absorbance using the formula ABS = 2 – log(%T) (Supplementary Materials, Table S4). The number of cells per ml was counted every other day from a single flask from the control group (No additional Se) using a hemocytometer (Supplementary Materials, Table S5). This was done to generate a standard curve to relate absorbance values to cell density values.
After 21 days, the Auxenochlorella were measured for final absorbance values and culture volumes of 200 mL were divided equally into four 50 mL centrifuge tubes. These tubes were each centrifuged at a speed of 8000 rpm for five minutes to remove algae from the water column. The excess media was decanted, and the Auxenochlorella for all four replicates of each treatment was amalgamated into a single tube. Treatments were amalgamated to reduce any replicate error that might affect the results of subsequent experiments, and to achieve sufficient biomass for subsequent experiments with the Aeolosoma. The resulting six tubes (one per treatment) were then filled with deionized water, the pellets were resuspended, and tubes were recentrifuged to rinse any remaining unincorporated Se and MeHg. The tubes were then left to dry in a fume hood with the caps removed. After drying, the pellet was weighed to determine differences in culture yield of Auxenochlorella and then set aside to use as a food source for the Aeolosoma in following experiments (Supplementary Materials, Table S6). We repeated the entire experiment a total of two times under identical conditions (Supplementary Materials, Table S7). However, for the second trial, the % transmittance of Auxenochlorella was measured every four days instead of every other day.
Experiment 2: Does dietary Se offer protection to Aeolosoma against exogenous MeHg in water?
The second experiment was done by feeding three different
Auxenochlorella food sources, differing in Se exposure during growth, to
Aeolosoma that were exposed to MeHg in water. The inference was that these different exposures would result in corresponding differences in Se content in
Auxenochlorella as a food source, though
Auxenochlorella was not directly tested for Se concentration due to limited biomass. An
Aeolosoma culture was obtained from Ward's Science (New York, USA) and they were fed with fish flakes prior to the experiment, while cultures were becoming established and the population was growing. The
Auxenochlorella food source for this experiment were the pellets harvested from the previous experiment (control groups). Four
Aeolosoma with developed posterior and anterior regions were placed into each of 16 35-mm microplates containing 4 ml dechlorinated tap water. Eight microplates received
Auxenochlorella grown with no additional Se, four microplates received
Auxenochlorella grown with low Se, and four microplates received
Auxenochlorella grown with high Se (
Table 2). Dishes were then covered with a clear lid to avoid external contamination and evaporation. On Day 4, a sublethal concentration of MeHg (2 µg L
−1) was added to all dishes except for four of the plates with
Auxenochlorella grown under no additional Se. These four plates (no additional dietary Se, no Hg) served as a reference for
Aeolosoma survival and reproduction.
The addition of MeHg was delayed until day 4 so that all Aeolosoma would have ingested Auxenochlorella, and where relevant, absorbed some of the dietary Se. The number of Aeolosoma per dish were counted every other day to determine differing patterns across Se treatments (Supplementary Materials, Table S8). They were also analyzed for immobility or other obvious behavioural changes, death and other unusual changes (e.g., pigmentation changes). The experiment ran for 12 days. On the 12th day, 0.5 mL of methanol was added to each dish as a fixative to ensure the final count was accurate with the growing numbers of Aeolosoma.
Experiment 3: Does Auxenochlorella treated with both MeHg and Se confer protection when consumed by Aeolosoma?
The third experiment was done by feeding
Aeolosoma the
Auxenochlorella grown under conditions of varying Se levels but consistent MeHg levels. Again, the assumption made was that
Auxenochlorella incorporated Se according to concentrations in the growth media; and that,
Aeolosoma, consequently, experienced variation in dietary Se:MeHg. The
Auxenochlorella that was used in this experiment was grown under conditions of: no additional Se and moderate MeHg (2 µg L
−1 MeHg), low Se and moderate MeHg, and high Se and moderate MeHg (
Table 3). The
Auxenochlorella food source for this experiment was the
Auxenochlorella pellets harvested from the first experiment (test groups), where MeHg and Se were added to the growth media. Similar to the previous experiment on
Aeolosoma, four
Aeolosoma were placed into each of 16 35-mm microplates. The
Aeolosoma were given dried
Auxenochlorella on Day 0 as a food source.
The number of Aeolosoma per dish was counted every other day (Supplementary Materials, Table S9). The Aeolosoma were observed for immobility or other obvious behavioural changes, death and other unusual changes (e.g., pigmentation changes). The experiment ran for 12 days. On the 12th day, 0.5 mL of methanol was added to each dish as a fixative to ensure the final count was accurate with the growing numbers of the Aeolosoma. This experiment and the previous Aeolosoma experiment were run concurrently, and the reference plates in Experiment 2, receiving Auxenochlorella grown with no additional Se and no Hg, served as a reference for both Experiments 2 and 3.
Data analyses
Experiment 1
A standard curve was used to convert absorbance values to
Auxenochlorella cell densities (
Appendix,
Fig. A1). Cell densities for each replicate vessel were plotted as a function of time using SigmaPlot (
Systat Software, Inc., Cary, NC) and fit to a 3-parameter sigmoidal curve based on least squares. The instantaneous growth rate (r
i) and the maximum density (k) were determined from the equation parameters of each curve and were extracted as response variables for statistical analysis of
Auxenochlorella growth response to Se and MeHg. The natural logarithm of each value for both r
i and k were calculated, and two values (i.e., standard deviation > 2, Cook's distance > 1) were examined individually. These values were implausibly high and did not correspond to high algal biomass, leading us to believe that this was a result of a data entry/calculation error. The model was run with and without the two values and the directional effect of the model was consistent. Ultimately, they were not removed as outliers. Treatment variables, MeHg and Se, were converted to factor variables and an additive two-way ANOVA model was run on the data for both trials of this experiment combined. This was done because a preliminary analysis showed no difference in growth parameters between trials. The models used were
ln ri = Se + MeHg + Se*MeHg + error for growth rate and
ln k = Se + MeHg + Se*MeHg + error for maximum density. Where the interaction term was not statistically significant, it was removed and the model was re-run. The simplified models used were
ln ri = Se + MeHg + error for growth rate and
ln k = Se + MeHg + error for maximum density. We assessed the normality of residuals using the Shapiro–Wilk test and
Q–
Q plots, applying log transformations when deviations from normality were detected. To test for homoscedasticity, we used the Breusch-Pagan test and visually inspected residuals versus fitted values plots. Residuals were examined to check for patterns and ensure that the assumption of homoscedasticity was met. These diagnostic checks helped confirm the robustness of our ANOVA results.
Experiments 2 and 3
Data were again analyzed using two-way ANOVA with MeHg and Se level as independent factors, recognizing that the experiment was not fully factorial with respect to MeHg. The
Aeolosoma counts from each plate were plotted versus time in SigmaPlot and the curves were fit to a three-parameter sigmoidal curve. As in the first experiment, the instantaneous growth rate (r
i) and the maximum density (k) were extracted from the fitted curves for statistical analysis. There were no outliers removed from these two experiments involving
Aeolosoma prior to running ANOVA. The models used were
ri = Se + MeHg + error for growth rate and
k = Se + MeHg + error for maximum density. Again, assumptions of the ANOVA models were rigorously tested to ensure the validity of our results with normality tests and tests for homoscedasticity as above. All statistical analyses for experiments 1, 2, and 3 were performed in R
v. 4.1.0 (
R Core Team, 2021) and RStudio
v. 4.1.0 (
R Studio Team, 2020) using the package Rcmdr
v. 2.7-1 (
Fox, 2017).
Discussion
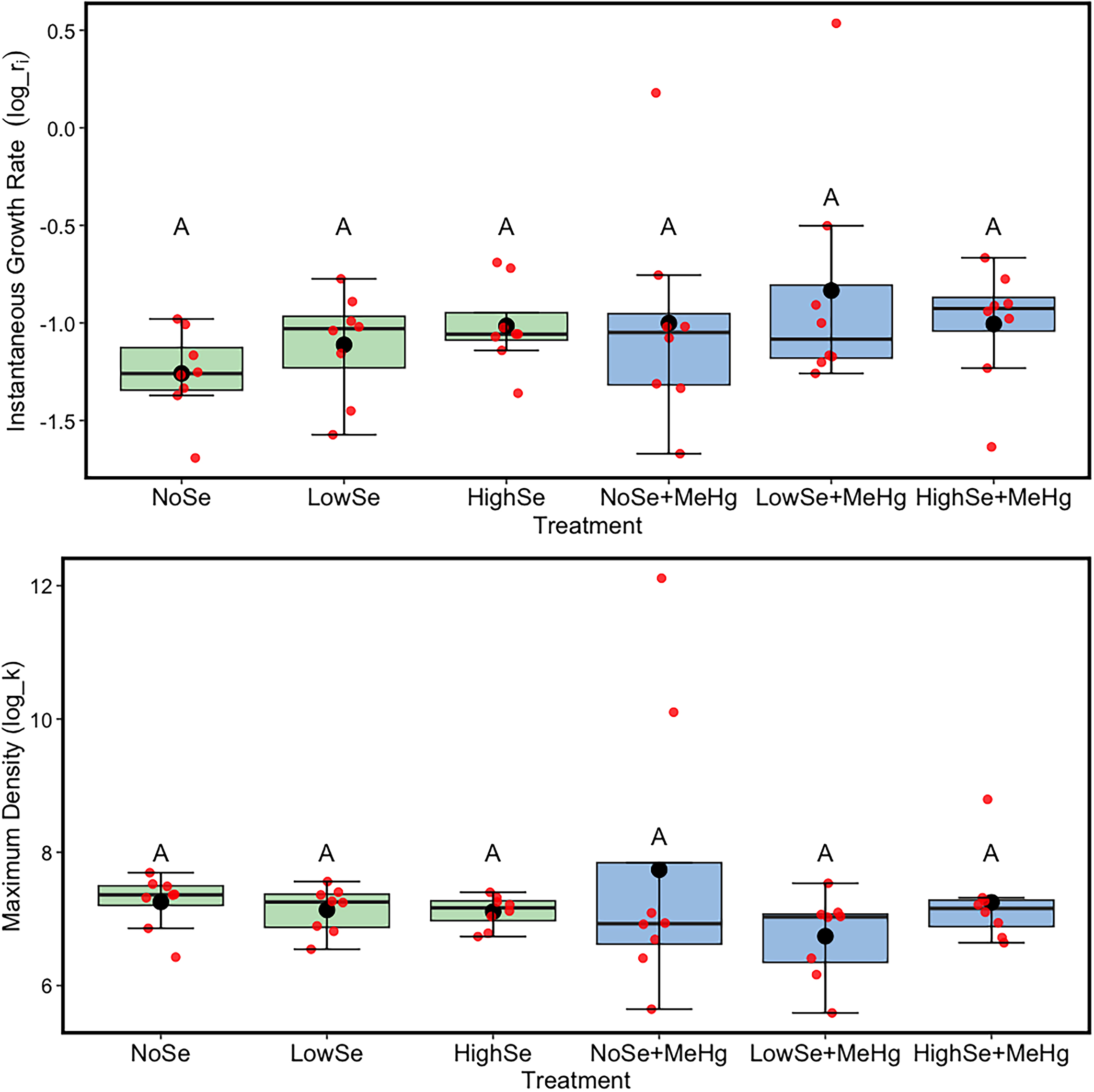
MeHg did not have a toxic impact on the
Auxenochlorella populations at the concentration used in our study as maximum density and instantaneous growth rate was not significantly reduced when the algae were exposed to MeHg in their growth medium (Experiment 1). This was not consistent with existing literature as
Rai et al. (1981) showed that MeHg reduced survival of
Chlorella vulgaris by 50% at 1 µg L
−1. The same study also showed that MeHg decreased the growth rate of
Chlorella. A decrease in the growth rate of
Chlorella following inorganic Hg exposure was noted by
Rosko and Rachlin (1977) but at a much higher concentration of 1.03 mg L
−1. The absence of a maximum density and growth rate decrease here might be due to
Auxenochlorella's ability to detoxify MeHg by forming chelates, consistent with studies showing
Chlorella's metal detoxification via phytochelatin production (
Gomez-Jacinto et al. 2015) and increased antioxidant enzymes (
Ajitha et al. 2019;
Leon-Vaz et al. 2021)
Se, like MeHg, did not have an impact on the growth rate of
Auxenochlorella at the levels used in our study. This aligns with Experiment 1’s hypothesis, which predicted that Se would exhibit a protective effect against MeHg's toxicity based on the outcome from a previous study (
Moreno et al. 2014). Se is a micronutrient in plants and was expected to stimulate growth as seen in a study done by
Sun et al. (2014) that showed increased
Chlorella biomass following exposure to Se at concentrations as high as 75 mg L
−1 (
Sun et al. 2014). The results seen in this study could be attributed to Chlorella's propensity to metabolize selenate to volatile dimethylselenide (
Neumann et al. 2003).
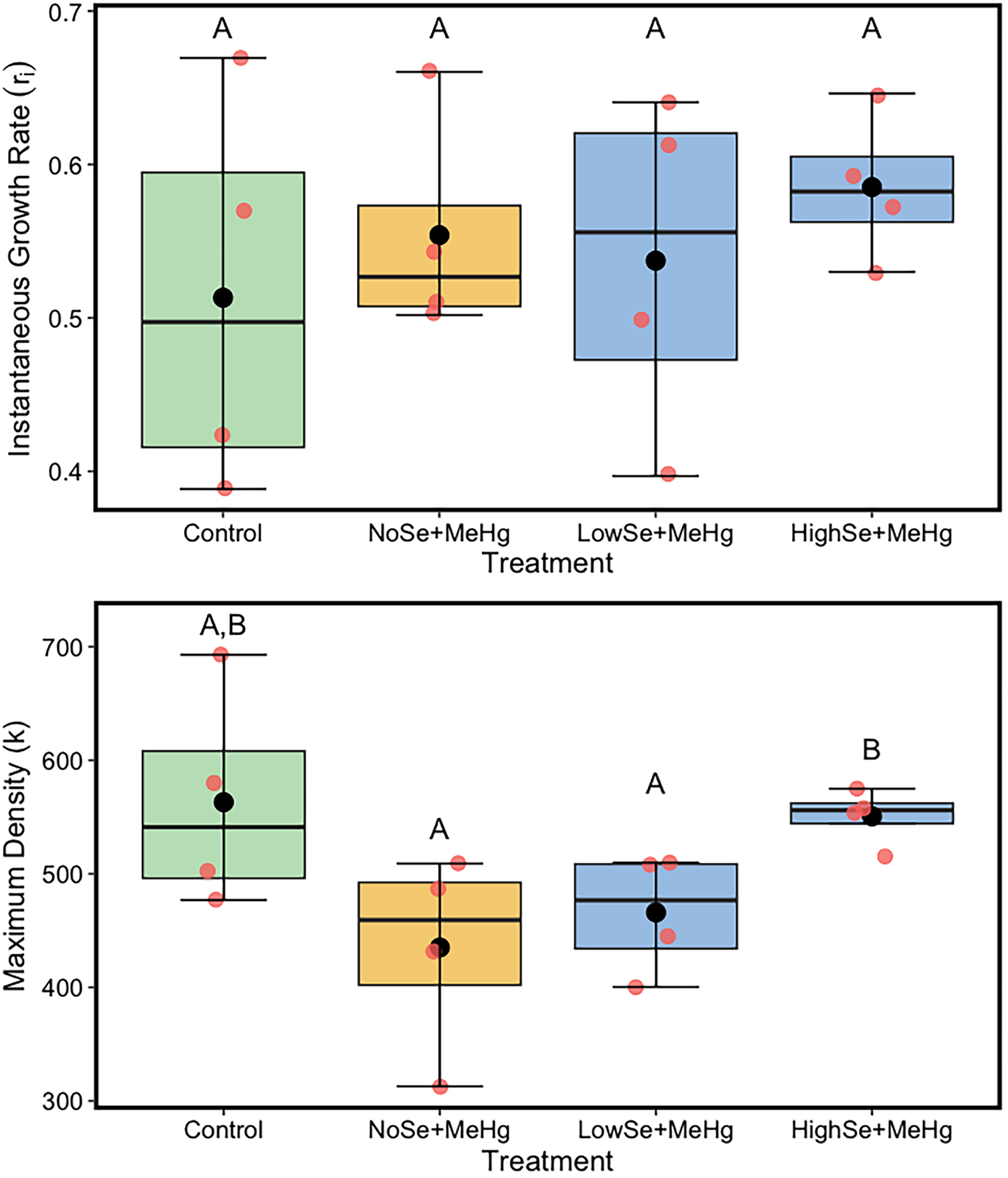
Waterborne MeHg and dietary Se had no significant impact on the instantaneous growth rate of
Aeolosoma populations, contrary to our hypothesis that exogenous MeHg would have a negative impact which should be offset by a positive impact from Se (Experiment 2). However, the negative impact of waterborne MeHg was evident when looking at the significant decrease in maximum density of
Aeolosoma populations when exposed to MeHg at a concentration of 2 µg L
−1. Consistent with Experiment 2’s hypothesis, this toxic effect of MeHg is well established based on previous studies performed on aquatic invertebrates (
Best et al. 1981;
Li et al. 2015).
Behavioural changes of the
Aeolosoma, including swimming in circles and flipping over rapidly, were observed between days 4 and 6 of Experiment 2. By Day 8 this behaviour ceased, potentially due to death or the tendency of MeHg to adsorb onto glassware (
Leermakers et al. 1990). Though only a few worms exhibited this behaviour, it is consistent with symptoms of toxicity induced by MeHg. Similar abnormal behaviours in animals were reported following the Hg contamination of the English-Wabigoon river system, where local residents reported cats salivating and stumbling in circles and turkey vultures flying in disordered patterns (
Shkilnyk 1985). A considerable amount of research documents Hg-induced changes in animal behaviour attributed to its toxicity to the central and peripheral nervous systems (
Chin et al. 2013;
Mora-Zamorano et al. 2016;
Malqui et al. 2018).
The toxicity of MeHg on
Aeolosoma populations in our study may have been exacerbated by the presence of dried
Auxenochlorella. In a few studies, dried
Chlorella adsorbed inorganic Hg to its surface (
Solisio et al. 2017;
Kumar et al. 2020), and various cellular functional groups (i.e., carboxyl, phosphoryl, amino, hydroxyl and carbonyl groups) found in
Chlorella have an affinity towards metal ions (
Vogel et al. 2010;
Edris et al. 2014;
Yadav et al. 2021). Although
Chlorella's adsorption capabilities may have potential in bioremediation, a propensity to adsorb inorganic Hg may also result in increased exposure when organisms ingest the
Chlorella.
As predicted, dietary Se displayed a significant protective effect on toxicity of both waterborne Hg and MeHg that was initially waterborne but may have become adsorbed to the food for
Aeolosoma maximum densities (Experiment 2). This protective effect is consistent with past research on various organisms that showed dietary Se mitigates MeHg toxicity from different exposure routes (
Turner and Swick 1983;
Bjerregaard et al. 1999;
Bjerregaard et al. 2011;
Rowe and Heyes 2017). The lower level of dietary Se was more effective in decreasing MeHg toxicity than the higher level of Se. Toxic levels of Se in aquatic invertebrates vary widely across species (
DeBruyn and Chapman 2007) and there is currently no data identifying the threshold of toxicity for Se in
Aeolosoma. Therefore, it is possible that the high level of Se transitioned from being beneficial to the
Aeolosoma to being an additional source of stress. Even so, populations feeding on the higher Se concentrations taken up by their food source (
Auxenochlorella) still had a significant rescue effect on the maximum density of
Aeolosoma populations in the presence of MeHg, indicating that the Se levels used in our study were not toxic.
Experiment 3 demonstrated that while dietary MeHg did not affect
Aeolosoma's instantaneous growth rate, there was a significant negative effect on growth rates from MeHg administered through the diet on maximum density. This dietary route of exposure is of greater ecological relevance than the primarily waterborne exposure in Experiment 2, as diet is the primary mode of entry for MeHg into food chains. For example, only 10% of MeHg accumulation in fish is from the water column (
Spry and Wiener 1991). The higher level of Se tested fully mitigated the toxic effect of diet-based MeHg uptake in
Aeolosoma as expected (Experiment 3) given the protective effect that Se has on the toxicity of Hg (
Turner and Swick 1983;
Belzile et al. 2006;
Ouedraogo et al. 2015).
On an ecosystem level, the results of experiments 1-3 indicate that MeHg, at an ecologically relevant, sublethal concentration that can occur in Hg contaminated ecosystems (
Gandhi et al. 2007), can decrease the maximum density or carrying capacity of
Aeolosoma because it acts as a stressor. Se levels used in this study may also occur in nature depending on external factors like sediment characteristics and anthropogenic influences (
May et al. 2008) The presence of toxins or chemical stressors in aquatic ecosystems is correlated with a decrease in carrying capacity (
Hendriks et al. 2005). This can be a result of various mechanisms induced by the toxin such as death, disease, reproductive failure, reduced food source, and reduced habitat (
Hendriks et al. 2005). The carrying capacity or maximum density of
Aeolosoma in our experiment were negatively impacted by MeHg, but instantaneous population growth rates remained unchanged in most cases. This indicates that reproductive failure was not occurring at a measurable scale. Instead, a higher death rate is likely the primary reason for the decreased carrying capacity or density. The constant growth rate despite decreased carrying capacity may also be a result of an early or delayed response of the organisms to MeHg. The growth rate across the entire duration of the experiments may not reflect rapid deaths or reproductive failure occurring at early or later stages of the experiment.
Decreasing the carrying capacity of organisms in an ecosystem can have ramifications all the way up the food chain. If MeHg is present in aquatic ecosystems and the density of primary producers and consumers at the base of the food chain decreases, a food source for all organisms at higher trophic levels is severely reduced.
Aeolosoma, a lower trophic level detritovore, contributes significantly to the food chain as they serve as prey for various organisms such as
Dugesia tigrina (flatworm planarians
), and
Hydra americana (
Singer 1977). The loss of significant food sources may affect populations of predators, which in turn affect predator-prey population dynamics throughout the food chain. The relationship between MeHg retention and food availability may also be a compounding impact with the decrease of food sources (
Park et al. 2009;
Madenjian et al. 2012). A study by
Park et al. (2009) found that MeHg concentrations in fish correlated with body weight, dietary habits and food availability, suggesting that the type and amount of food consumed directly impact MeHg accumulation. In addition to this, overall bioaccumulation in the food web may be increased when there is less food available as there is less biomass to dilute MeHg concentrations thereby resulting in higher concentrations (
Karimi et al. 2007).
Further studies can build on our research to explore whether this concept extends to secondary or tertiary consumers. Moreover, additional research may improve these results by addressing some of the research limitations. The inability to determine actual exposure concentrations throughout the experiments represents a limitation in our study as adsorption of MeHg on the glassware and volatilization of dimethylselenide could not be accounted for. In addition to this, due to the microscopic size of Aeolosoma, performing any physical analysis on the accumulation or removal of MeHg when it was co-introduced alongside Se was not possible. Testing a wider range of concentrations of MeHg and Se on these organisms could also provide more insight into the thresholds of MeHg and Se toxicity at the base of the food chain as there is no current research addressing this. Lastly, further research could investigate the potential impacts of MeHg on Aeolosoma development, specifically head resorption, which is the mechanism of asexual reproduction exhibited in these worms. This is relevant as there was an apparent lower number of mature worms noted at the end of Experiment 2 and 3, though it was not quantified or investigated further.
Our results make a small contribution towards understanding the interaction between MeHg and Se across trophic levels in freshwater ecosystems. As previously discussed, there is a gap in understanding the role of trophic transfer in Se's protection against MeHg. But this is an important area of research because both MeHg and Se are usually transferred through the food web and both can bioaccumulate and biomagnify. Typically, not a lot of free MeHg or Se resides in the water column so for most animals the primary route of exposure will be through dietary intake. Understanding whether ingesting both Se and MeHg through a dietary source allows for trophic transfer of protection from MeHg toxicity could be crucial knowledge necessary for remediating legacy Hg contamination in both urban and non-urban areas.