Seasonal patterns and key chemical predictors of dissolved greenhouse gases in small prairie pothole ponds
Abstract
Introduction
Methods
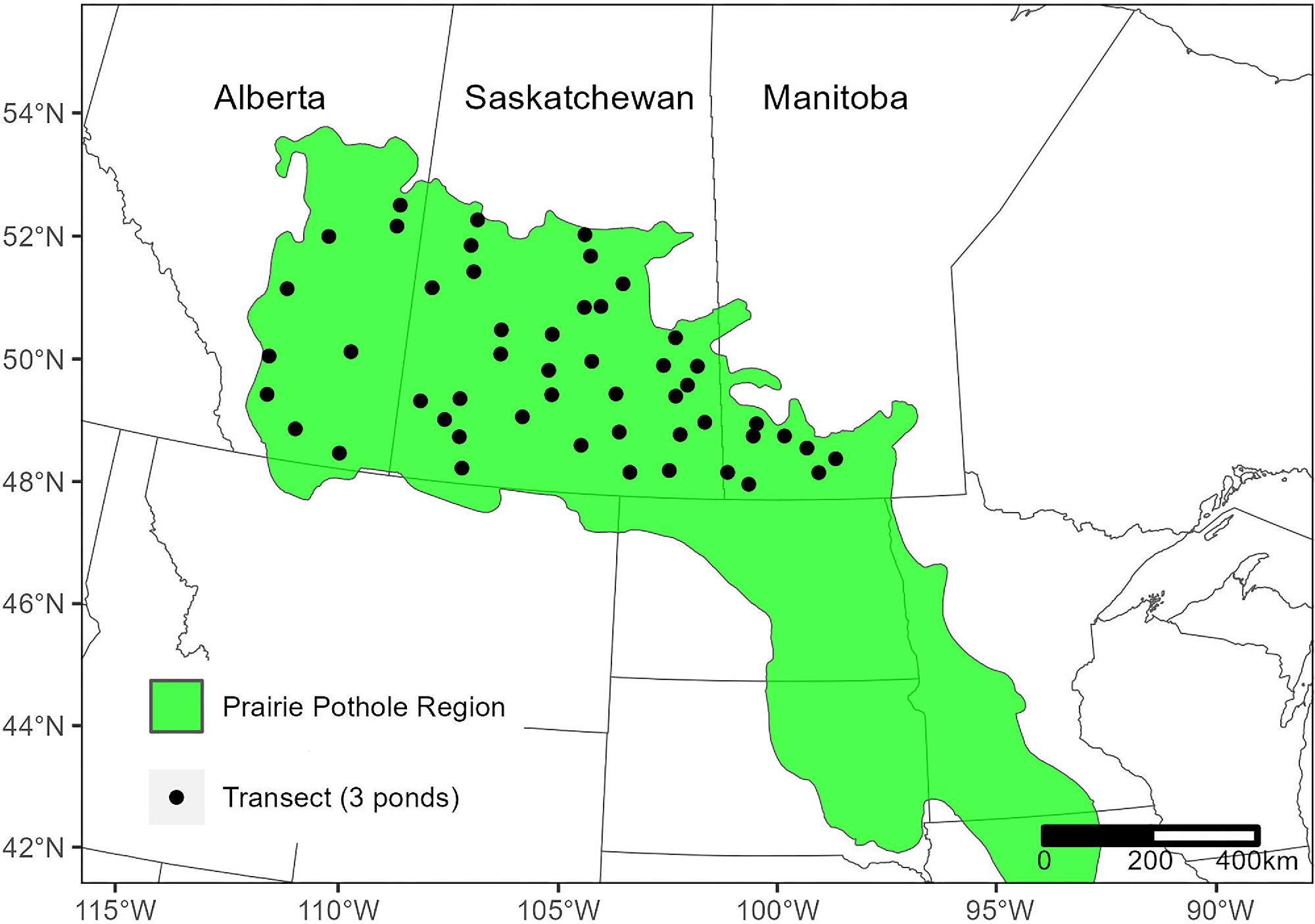
Study area and field survey
Laboratory analysis
GHG calculations
Statistical analysis
Results
Pond physicochemical conditions
| Parameter | n | Unit | Min. | Mean | Median | Max. |
|---|---|---|---|---|---|---|
| Alkalinity | 275 | mg L−1 | 28 | 250 | 200 | 760 |
| Chl a | µ | µg L–1 | 0 | 17 | 7.0 | 210 |
| Cond. | 256 | µS cm−1 | 90 | 1200 | 770 | 7300 |
| pH | 255 | 6.7 | 7.8 | 8.2 | 11 | |
| DOC | 259 | mg L−1 | 9.8 | 33 | 30 | 140 |
| SO42− | 277 | mg L−1 | 7.0 | 460 | 150 | 5500 |
| TP | 276 | mg L−1 | 0.018 | 0.74 | 0.46 | 4.4 |
| TDP | 277 | mg L−1 | 0.006 | 0.6 | 0.34 | 4.4 |
| SRP | 211 | mg L−1 | 0.0028 | 0.51 | 0.28 | 4.4 |
| TN | 274 | mg L−1 | 0.26 | 3 | 2.6 | 22 |
| Urea | 278 | mg L−1 | 0.01 | 0.081 | 0.06 | 1.3 |
| TAN | 276 | mg L−1 | 0.0036 | 0.049 | 0.016 | 1.9 |
| NOx− | 258 | mg L−1 | 0.058 | 0.24 | 0.21 | 0.89 |
| Sediment TC | 122 | % weight | 1.5 | 8.3 | 6.7 | 29a |
| Sediment IC | 116 | % weight | 0 | 0.16 | 0 | 2.4 |
| Sediment OC | 117 | % weight | 1.7 | 8.9 | 7.1 | 30a |
| Sediment clay | 119 | % | 1.6 | 3.7 | 3.5 | 8.4 |
| Sediment silt | 119 | % | 34 | 66 | 65 | 87 |
| Sediment sand | 119 | % | 8.5 | 31 | 31 | 62 |
| Surface area | 194 | m2 | 28 | 2600 | 1000 | 19 000 |
| Twater | 279 | °C | 1.6 | 14 | 14 | 31 |
| Wind speed | 242 | m s−1 | 0 | 2.0 | 1.8 | 6.2 |
| δ2H | 48 | ‰ | −200 | −130 | −140 | −58 |
| δ18O | 48 | ‰ | −24 | −15 | −15 | −0.88 |
| d-excess | 48 | ‰ | −51 | −15 | −15 | 5.1 |
| ΔCH4 | 246 | µmol L−1 | 0.0070 | 4.0 | 0.72 | 120 |
| ΔCO2 | 242 | µmol L−1 | −26 | 87 | 8.4 | 1100 |
| ΔN2O | 261 | µmol L−1 | −0.010 | 0.00056 | −0.0013 | 0.24 |
Note: Parameters are alkalinity, chlorophyll a (chl a), specific conductance (Cond.), pH, dissolved organic carbon (DOC), sulfate (SO42−), total phosphorus (TP), total dissolved phosphorus (TDP), soluble reactive phosphorus (SRP), urea, total ammonia nitrogen (TAN), total oxidised nitrogen (NO x−: nitrate (NO3− ) + nitrite (NO2− )), sediment total carbon (TC), inorganic carbon (IC), organic carbon (OC), clay, silt and sand, water temperature ( Twater), hydrogen and oxygen isotope ratios (δ2H and δ18O), and deuterium-excess (d-excess). ΔGHG concentrations are the difference between dissolved concentrations and theoretical conditions under equilibrium with the atmosphere for methane (CH4), carbon dioxide (CO2), and nitrous oxide (N2O).
Pond GHGs
| GHG | Season | na | Supersaturated (%) | Saturated (%) | Undersaturated (%) |
|---|---|---|---|---|---|
| CH4 | Spring | 140 | 100 | 0 | 0 |
| Summer | 107 | 100 | 0 | 0 | |
| CO2 | Spring | 135 | 63.0 | 3.0 | 34.0 |
| Summer | 108 | 51.8 | 2.8 | 45.4 | |
| N2O | Spring | 144 | 18.0 | 22.9 | 59.0 |
| Summer | 118 | 14.4 | 14.4 | 71.2 |
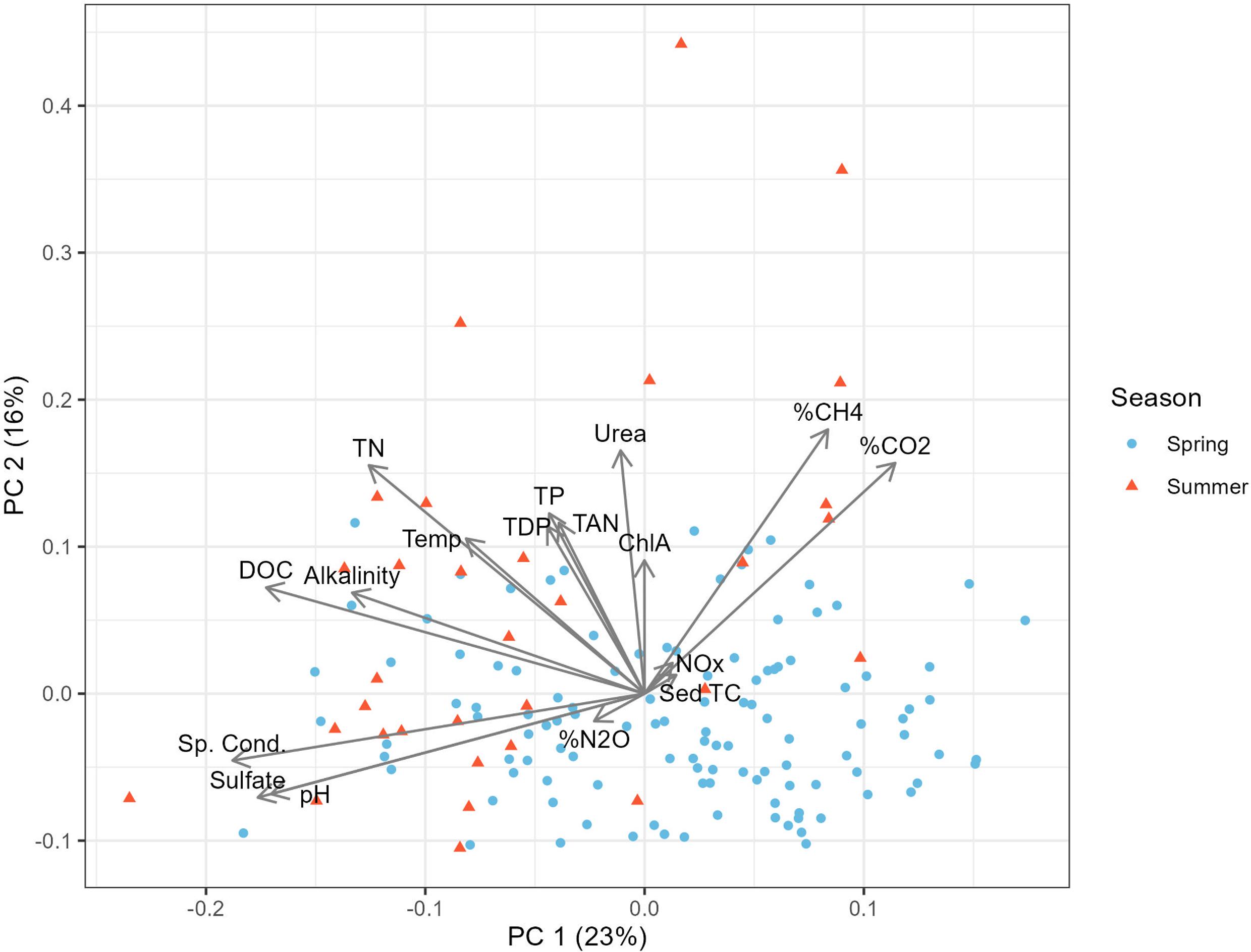
Physicochemical predictors of GHGs
| %CH4 | %CO2 | %N2O | |
|---|---|---|---|
| ΔCH4 | 1.0** | 0.51** | −0.56** |
| ΔCO2 | 0.51** | 0.99** | −0.44** |
| ΔN2O | −0.53** | −0.45** | 0.98** |
| Alkalinity | −0.01 | −0.03 | −0.08 |
| Chl a | 0.19** | 0.06 | −0.04 |
| Cond. | −0.48** | −0.33** | 0.17* |
| DOC | 0.0 | −0.21** | −0.08 |
| NOx− | 0.14* | 0.14* | 0.02 |
| pH | −0.32** | −0.8** | 0.28** |
| Sed TC | −0.06 | 0.17* | −0.05 |
| SO42− | −0.59** | −0.39** | 0.29** |
| TAN | 0.2** | −0.03 | −0.1 |
| TDP | 0.07 | 0.1 | −0.01 |
| TP | 0.13 | 0.11 | −0.05 |
| TN | 0.1 | −0.18* | −0.05 |
| Urea | 0.22** | 0.24** | −0.15* |
| Twater | 0.32** | −0.09 | −0.17* |
Note: Correlations between physicochemical variables were analysed, but coefficients were not included here (Supplementary Table S4). Variable abbreviations are as follows: CH4, methane; CO2, carbon dioxide; N2O, nitrous oxide saturation (i.e., the difference between observed and theoretical equilibrium conditions) as % (%) and delta values (Δ); Chl a, chlorophyll a; Cond, specific conductance; DOC, dissolved organic carbon; NOx−, total oxidised nitrogen (NO3−, nitrate + NO2−, nitrite); Sed TC, sediment total carbon; SO42−, sulfate; TAN, total ammonia nitrogen; TDP, total dissolved phosphorus; TP, total phosphorus; TN, total nitrogen; Twater, water temperature.
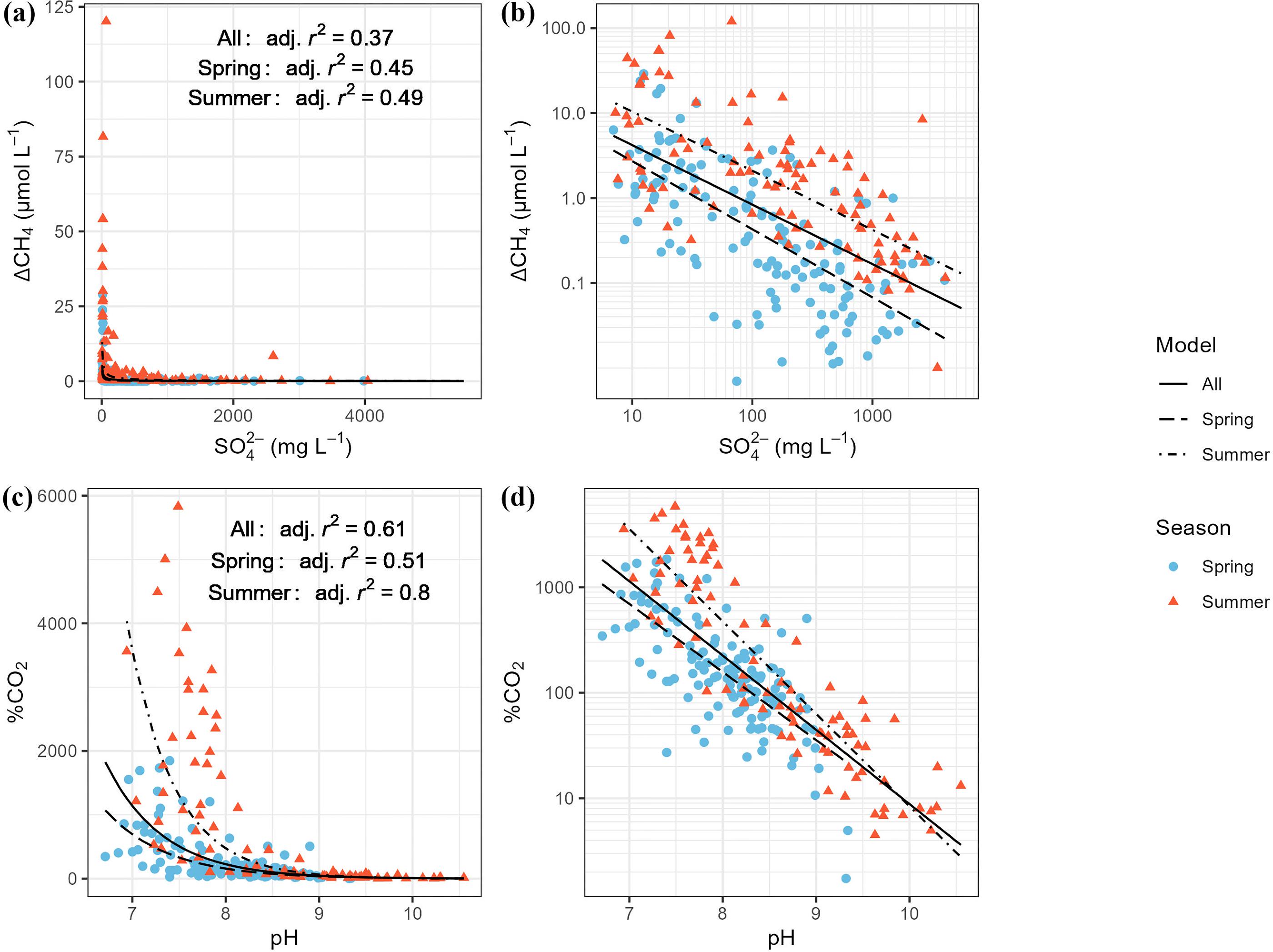
| Variable pair | Function | Adjusted R2 | p-value |
|---|---|---|---|
| ΔCH4–SO42− | Power | 0.36 | <0.001 |
| ΔCH4–pH | 2nd order polynomial | 0.17 | <0.001 |
| ΔCH4–Twater | Exponential | 0.07 | <0.001 |
| %CO2–pH | Exponential | 0.61 | <0.001 |
| %CO2–SO42− | Power | 0.14 | <0.001 |
| %CO2–Urea | Linear | 0.14 | <0.001 |
| %N2O–pH | Exponential | 0.05 | <0.001 |
| %N2O–SO42− | Power | 0.06 | <0.001 |
Note: ΔGHG data were used for CH4, otherwise %GHG data were used to avoid negative values. For each variable pair, we tested different functions to describe the relationship. Displayed in this table is the model for each pair with the highest adjusted R2 that had significant p-values for the coefficients. Variable abbreviations are as follows: ΔCH4, methane saturation; %CO2, carbon dioxide saturation; %N2O, nitrous oxide saturation; SO42−, sulfate; Twater, water temperature.
Seasonal differences
| Variable | FDR p-value | Mean difference (paired t-test) | Median difference (signed-rank test) | Overall median (minimum, maximum) | Units |
|---|---|---|---|---|---|
| Alk. | <0.001 | 65 | 200 (28, 760) | mg L−1 | |
| Chl a | 0.51 | 0.19 | 7 (0, 210) | µg L−1 | |
| Cond. | <0.001 | 270 | 770 (90, 7300) | µS cm−1 | |
| DOC | <0.001 | 8.9 | 30 (9.8, 140) | mg L−1 | |
| NOx | 0.021 | 0.0027 | 0.016 (0.0036, 1.9) | mg L−1 | |
| pH | <0.001 | 0.56 | 8.2 (6.7, 11) | ||
| SO42− | <0.001 | 30 | 150 (7, 5500) | mg L−1 | |
| TAN | <0.001 | 0.036 | 0.06 (0.01, 1.3) | mg L−1 | |
| TDP | <0.001 | 0.095 | 0.34 (0.006, 4.4) | mg L−1 | |
| TP | 0.0011 | 0.13 | 0.46 (0.018, 4.4) | mg L−1 | |
| TN | <0.001 | 1.1 | 2.6 (0.26, 22) | mg L−1 | |
| Urea | <0.001 | 0.036 | 0.21 (0.058, 0.89) | mg L−1 | |
| Twater | <0.001 | 12 | 14 (1.6, 31) | °C | |
| δ2Ha | <0.001 | 34 | −140 (–200, –58) | ‰ | |
| δ18Oa | <0.001 | 5.2 | −15 (–24, –0.88) | ‰ | |
| d-excessa | 0.027 | −7.2 | −15 (–51, 5.1) | ‰ | |
| Wind | 0.077 | 0.33 | 1.8 (0, 6.2) | m s−1 | |
| ΔCH4 | <0.001 | 0.55 | 0.72 (0.007, 120) | µmol L−1 | |
| ΔCO2 | 0.023 | 3.7 | 8.6 (–26, 1100) | µmol L−1 | |
| ΔN2O | 0.0037 | −0.00074 | −0.0013 (–0.01, 0.24) | µmol L−1 | |
| FluxCH4 | <0.001 | 0.88 | 0.41 (0.0027, 100) | mmol m−2 d−1 | |
| FluxCO2 | 0.026 | −2.1 | 5 (–38, 710) | mmol m−2 d−1 | |
| FluxN2O | <0.001 | −1.3 | −0.9 (–11, 420) | µmol m−2 d−1 |
Note: Variables with differences between pairs that were severely non-normal were analysed with the non-parametric signed-rank test. Positive mean or median differences indicate higher summer values.
Gas exchange with the atmosphere
| Parameter | GWP | n | Unit | Min. | Mean | Median | Max. |
|---|---|---|---|---|---|---|---|
| FluxCO2 | 207 | mmol m−2 d−1 | −38 | 52 | 5.0 | 710 | |
| FluxCH4 | 221 | mmol m−2 d−1 | 0.0027 | 2.8 | 0.41 | 100 | |
| FluxN2O | 184 | µmol m−2 d−1 | −11 | 1.9 | −0.90 | 420 | |
| FluxCO2 | 207 | g CO2 m−2 d−1 | −1.7 | 2.3 | 0.22 | 31 | |
| Spring | 114 | g CO2 m−2 d−1 | −0.4 | 1.0 | 0.26 | 12 | |
| Summer | 93 | g CO2 m−2 d−1 | −1.7 | 3.9 | 0.036 | 31 | |
| FluxCH4, CO2-eq | GWP100 | 221 | g CO2 m−2 d−1 | 0.0012 | 1.2 | 0.18 | 45 |
| Spring | 122 | g CO2 m−2 d−1 | 0.0012 | 0.37 | 0.079 | 5.7 | |
| Summer | 99 | g CO2 m−2 d−1 | 0.0025 | 2.2 | 0.38 | 45 | |
| FluxCH4, CO2-eq | GWP20 | 221 | g CO2 m−2 d−1 | 0.0035 | 3.5 | 0.52 | 130 |
| Spring | 122 | g CO2 m−2 d−1 | 0.0035 | 1.1 | 0.23 | 17 | |
| Summer | 99 | g CO2 m−2 d−1 | 0.0074 | 6.5 | 1.1 | 130 | |
| FluxN2O, CO2-eq | 184 | g CO2 m−2 d−1 | −0.14 | 0.023 | −0.011 | 5.1 | |
| Spring | 95 | g CO2 m−2 d−1 | −0.053 | 0.0055 | −0.0063 | 0.47 | |
| Summer | 89 | g CO2 m−2 d−1 | −0.14 | 0.041 | −0.017 | 5.1 | |
| Total, CO2-eq | GWP100 | g CO2 m−2 d−1 | −1.8 | 3.5 | 0.39 | 81 | |
| Spring | g CO2 m−2 d−1 | −0.45 | 1.4 | 0.33 | 18 | ||
| Summer | g CO2 m−2 d−1 | −1.8 | 6.1 | 0.40 | 81 | ||
| Total, CO2-eq | GWP20 | g CO2 m−2 d−1 | −1.8 | 5.8 | 0.73 | 170 | |
| Spring | g CO2 m−2 d−1 | −0.45 | 2.1 | 0.48 | 29 | ||
| Summer | g CO2 m−2 d−1 | −1.8 | 10 | 1.1 | 170 |
Note: Spring and summer CO2-eq fluxes are also shown. Total values summarize the sum of CO2-eq fluxes from ponds with data for all three GHGs to estimate total GHG contribution to the atmosphere from pond surface water. To obtain CO2-eq, fluxes were converted to g m−2 d−1 and multiplied by the global warming potential metric with 20- and 100-year time horizons (CH4: GWP20 = 79.7 and GWP100 = 27.0. N2O: GWP20 = 273 and GWP100 = 273, Table 7.15 in Forster et al. 2021).
Discussion
Pond variability
Seasonal change
Pond GHGs and physicochemical predictors
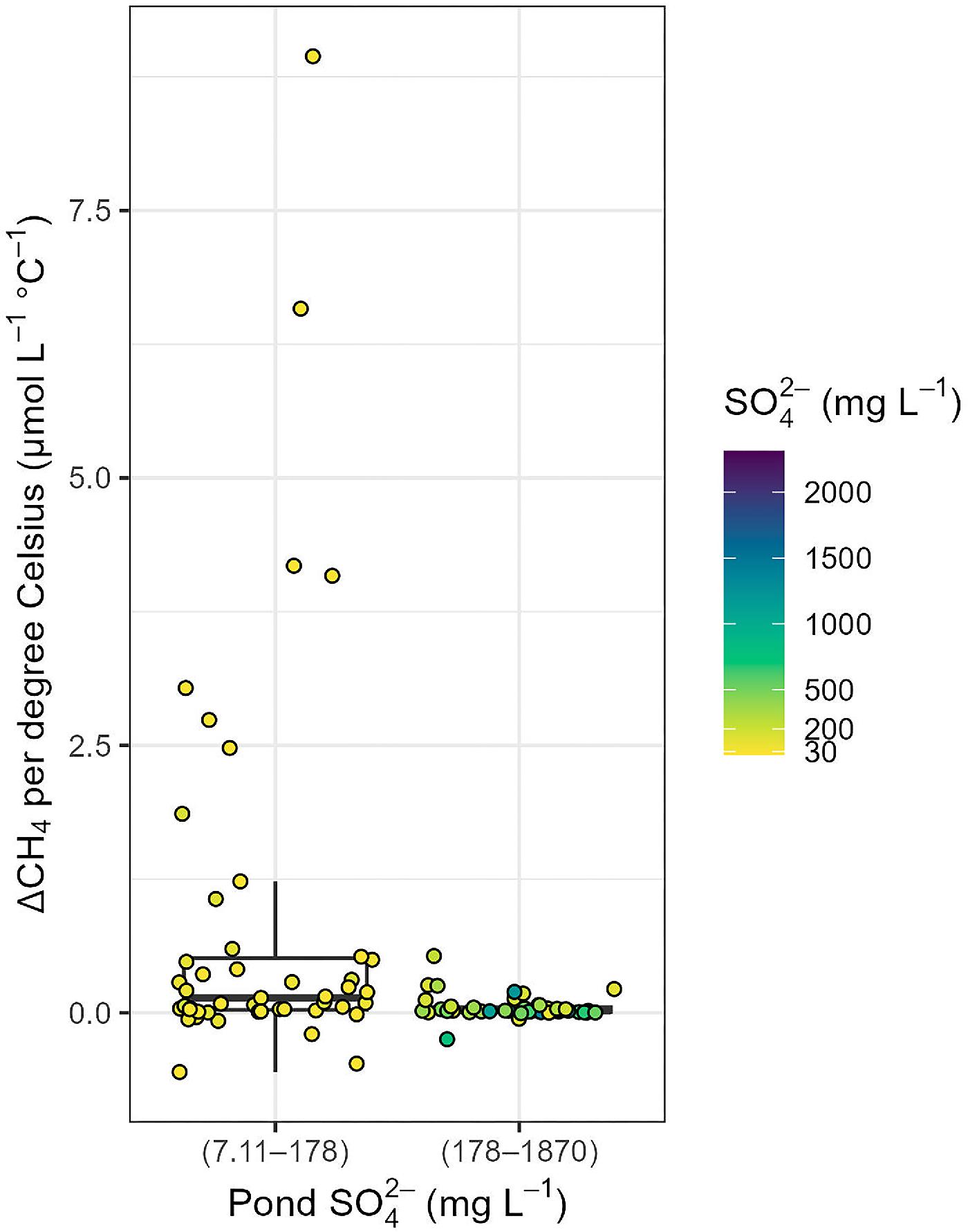
Methane
Carbon dioxide
Nitrous oxide
GHG fluxes and the radiative balance
| Mean diffuse fluxes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Flux source | Location | Sampling year (s) | Sites (n) | Surface areac (m2) | Study type | N2O (µmol m−2 d−1) | CO2 (mmol m−2 d−1) | CH4 (mmol m−2 d−1) | Flux method | Study |
| Pothole pond | Alberta, Saskatchewan, Manitoba, CA | April/May, June/July 2019 | 157 | 28–1900 | survey | 1.9 | 52 | 2.8 | Headspace equilibrium, k from wind model | This study |
| Pothole pond | Saskatchewan, CA | May–September, 2013 | 3 | 2670–8750 | intensive | NA | 83 | 4.9 | Headspace equilibrium | Bortolotti et al. (2016a) |
| Pothole pond | North Dakota, US | May–October, 2015 | 2 | intensive | NA | 60 | 7.0 | Static chamber | Dalcin Martins et al. (2017) | |
| Pothole pond | Montana, N. Dakota, S. Dakota, Minnesota, Iowa, US | April–September 2003–2016 | 177 (ranging from 12 to 119 each year) | 40 000–190 000 (incl. upland) | intensive | 1.0 | NA | NA | Static chamber | Tangen and Bansal (2022) |
| Pothole basina | Alberta, Saskatchewan, Manitoba, CA | April–October, 2004–2005 | 62 | NA | survey + intensive | 9.9 | NA | 1.5 | Static chamber | Badiou et al. (2011) |
| Pothole basinb | North Dakota, Minnesota, Iowa, US | April–September, 2005–2008 | 119 (with 41 sites for 2007–08) | 2100–18800 | intensive | 14 | NA | 69 | Static chamber | Tangen et al. (2015) |
| Small agricultural reservoir | Saskatchewan, CA | July–August, 2017 | 101 | 158–13900 | survey | 1.5 | 44 | 7.1 | Headspace equilibrium, measured k | Webb et al. (2019a, 2019b) |
| Global pond/lake estimate | Ponds | Various studies | 50 | <1000 | review | NA | 35 | 2.3 | Headspace equilibrium, k from lake-size | Holgerson and Raymond (2016) |
| Global pond/lake estimate | Ponds | Various studies | 22 | 1000–10000 | review | NA | 21 | 0.65 | Headspace equilibrium, k from lake-size | Holgerson and Raymond (2016) |
Note: We include one global estimate of CO2 and CH4 fluxes for additional context. Differences between GHG flux estimates can be caused by different methods and study designs.
Conclusions
Acknowledgements
References
Supplementary material
- Download
- 265.29 KB
Information & Authors
Information
Published In

History
Notes
Copyright
Data Availability Statement
Key Words
Sections
Subjects
Plain Language Summary
Authors
Author Contributions
Competing Interests
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
