Introduction
Competition's role in community assembly, diversification, and ecosystem functioning is widely acknowledged (
Estes et al. 2011). However, while numerous metrics have been developed for quantifying aspects of competition, it remains difficult to robustly identify the effects of competition in natural systems (
Weigelt and Jolliffe 2003). One observation that can provide support for the presence of interspecific competition is the difference in the realized ecological niche of allopatric and sympatric individuals (
Brown and Wilson 1956;
Losos 2000). The realized niche describes the environmental conditions and resources being used by a given organism or population and is encompassed by the fundamental niche, which includes all environmental conditions and resources that could potentially support that same organism or population (
Hutchinson 1965).
The confinement of organisms to restricted realized niches, in comparison to their broader fundamental niches, is thought to be a product of interspecific competition (
Roughgarden 1973). Organisms relying on distinct resource pools are unhindered by niche similarity and may occupy identically realized niches if it maximizes their fitness. However, resource limitation necessitates that the ecological niche of sympatric organisms be sufficiently differentiated to permit coexistence (
Hardin 1960;
MacArthur and Levins 1967a). Organisms altering aspects of their ecological niche in sympatry are said to be undergoing niche, or character, displacement (
Pfennig and Pfennig 2009). Because competition generally reduces fitness, niche displacement may be the result of selection for phenotypes that minimize interspecific competition (
Kingsolver and Pfennig 2007). Unfortunately, due to the myriad of covarying biological and environmental factors generating spatiotemporal variation in realized niches, competition's role cannot be constrained by observing niche differentiation per se. Nonetheless, inferences such as these are valuable when dealing with species for which controlled manipulation studies are infeasible (
Salsamendi et al. 2012).
Predators are important regulators of ecosystem function and community composition (
Prugh et al. 2009). For this reason, the outcome of competitive interactions among predators can have marked consequences for ecological communities (
Ritchie and Johnson 2009). Anthropogenic trends in climate and land-use have increased niche overlap and are intensifying competition among carnivores (
Manlick and Pauli 2020). Moving forward, efforts to characterise the role of competitive interactions in shaping the realized niche of carnivores will be critical for effective management of these species.
One carnivore of conservation concern is the Canada lynx,
Lynx canadensis (Kerr 1792); hereafter, “lynx” (
Koen et al. 2014). Currently, lynx occupy the majority of their historic range (∼95% of historic) in Canada but have declined (∼40% of historic) throughout their southern range and have been listed for protection in New Brunswick (“Of Concern” under the NB Species at Risk Act), Nova Scotia (“Endangered” under the NS Endangered Species Act), and the United States (”Threatened” under the US Endangered Species Act) (
Poole 2003;
Laliberte and Ripple 2004). In contrast, bobcats,
Lynx rufus (Schreber 1777), seem to be expanding their range in North America (
Kelly et al. 2016). Recent surveys indicate that bobcat populations are generally increasing or stable surrounding their contact zone with lynx in southern Canada, and viable lynx-bobcat hybrids have been documented (
Homyack et al. 2008;
Popescu et al. 2021).
Owing to their biological similarity, the space and resource requirements of bobcat and lynx are assumed to be quite similar (
Marrotte 2020). Therefore, the potential for competition between them is assumed to be high (
MacArthur and Levins 1967b). Competition with bobcats may be responsible for lynx range contractions, but present patterns of coexistence are thought to be maintained by habitat partitioning (
Parker et al. 1983;
Peers et al. 2013). Specifically, lynx are thought to make disproportionate use of habitats with increased snow cover and depth (
Peers et al. 2013). Unfortunately for lynx, snow is becoming more scarce across most of their southern range. In southern New Brunswick, the number of black ground days (no snow on the ground at meteorological stations) increased by 0.3 days/year between 1963 and 2022 (
Zhiwen 2022). If snow conditions spatially partition bobcat and lynx, then climate change is expected to increase contact and potentially competition, among them.
One potential difference between bobcat and lynx is that lynx are thought to exhibit a greater degree of specialization than bobcats (
Peers et al. 2012). Lynx at the northern extent of their range are well-known specialist predators of the snowshoe hare,
Lepus americanus (Erxleben 1777) (
Breitenmoser et al. 1993). Although southern lynx consume a wider variety of prey, snowshoe hares remain the primary prey throughout their range (
Roth et al. 2007;
Peers et al. 2014). Specialist behaviors may be favored in the environments lynx currently occupy, but reliance on snowshoe hare likely contributes to the severity of lynx population collapse during periods of low hare abundance (
Peers et al. 2014). Bobcats, on the other hand, have been documented consuming a wide variety of prey—including snowshoe hare when available (
Litvaitis and Harrison 1989;
Gobin et al. 2022). Generalist behaviors in bobcats could potentiate their ongoing range expansions and make them superior competitors in increasingly altered and heterogeneous landscapes (
Kassen 2002).
The multi-dimensional nature of ecological niches makes them difficult to comprehensively describe. However, important niche axes like the proportions of different prey in the diet can be inferred using stable isotope analysis (
Newsome et al. 2007). Because stable isotope information is integrated into tissues over a time period that is proportional to the turnover time of that tissue, simultaneously analyzing relatively long and short turnover tissues from the same organism yields information on resource use over different scales (
Tieszen et al. 1983;
Vander Zanden et al. 2015). Commonly analyzed tissues include the liver, which integrates a period of approximately one month, and muscle, which integrates a period of approximately two to three months (
Sponheimer et al. 2006). In our study, wild cats were primarily sampled during the late autumn and winter. Therefore, muscle is likely to reflect behavior in the late-autumn and early-winter, whereas the liver would be more reflective of winter behavior. When the estimates of prey use from liver and muscle are well correlated, it follows that resource use has varied little in the preceding months. Conversely, when estimates from liver and muscle disagree, it indicates that resource use is relatively more heterogeneous over time, which decreases the presumed prevalence of individual specialization.
To associate observed niche displacement among allopatric and sympatric populations with a novel competitive stress, our primary interpretative framework assumes that interspecific competition among our study species is trivial in allopatry. Moreover, we assume unidirectional expansion of bobcats into lynxes’ range, or vice versa, to be underlying sympatry (i.e., ranges are coming together and not separating). If this assumption is relaxed, it is also informative to consider how bobcat and lynx responded to the relaxation of interspecific competition, moving from sympatry into allopatry. Under these circumstances, observing niche displacement could be construed as evidence for the occurrence of ecological release. Ecological release refers to niche expansions and shifts when interspecific competition is relaxed. Ecological release is thought to occur due to the adaptive advantage inherent to broad ecological niches at the population level (
Herrmann et al. 2021). Although empirical evidence is mixed, multiple forms of ecological release have been described depending on whether the population's increased niche breadth stems from decreased interindividual niche similarity (e.g., niche variation hypothesis;
Van Valen 1965) or decreased individual specialization (e.g., parallel release hypothesis;
Bolnick et al. 2010).
Our goal was to characterize competition among bobcat and lynx by investigating whether niche displacement occurs in lynx, bobcat, or both species near their range contact zone. To accomplish this, we estimated resource use by analyzing stable isotopes of carbon and nitrogen in muscle and liver sampled from allopatric and sympatric populations of bobcat and lynx living in New Brunswick, Canada. Our primary hypothesis was that competition among bobcat and lynx would lead to observable isotopic niche displacement in one, or both, species. We were also interested in testing whether bobcat and lynx occupy opposite ends of the specialist-generalist spectrum with respect to their prey selection. We anticipated finding a smaller isotopic niche, and greater agreement between liver and muscle data, in populations exhibiting a greater degree of specialization.
Methods
Sample collection
Our study was conducted during the winter trapping seasons of 2016–17 and 2017–18. Bobcat and lynx samples were originally collected by trappers licensed in New Brunswick using devices approved in accordance with international humane trapping standards. As part of New Brunswick's furbearer management practices, trappers must supply bobcat carcasses to the New Brunswick Department of Natural Resources and Energy Development (DNRED). Furthermore, as lynx are a protected species, all dead lynx found must be presented to the DNRED. Lynx were predominantly killed by vehicular strikes (n = 16) or incidentally caught in traps or snares set for coyote or bobcat (n = 61). Most wild cats were killed between November and February each year, but a small number of lynx (n = 10) were obtained in May, August, or September. The date of capture and wildlife management zone (WMZ) from which each animal was collected were recorded, along with the body mass and sex of the individual when possible. Notably, bobcat weights were measured after the removal of the pelt, while lynx carcasses were weighed whole. Representative subsamples of lynx (n = 66) and bobcat (n = 82) collected by DNRED were frozen until dissection and analysis.
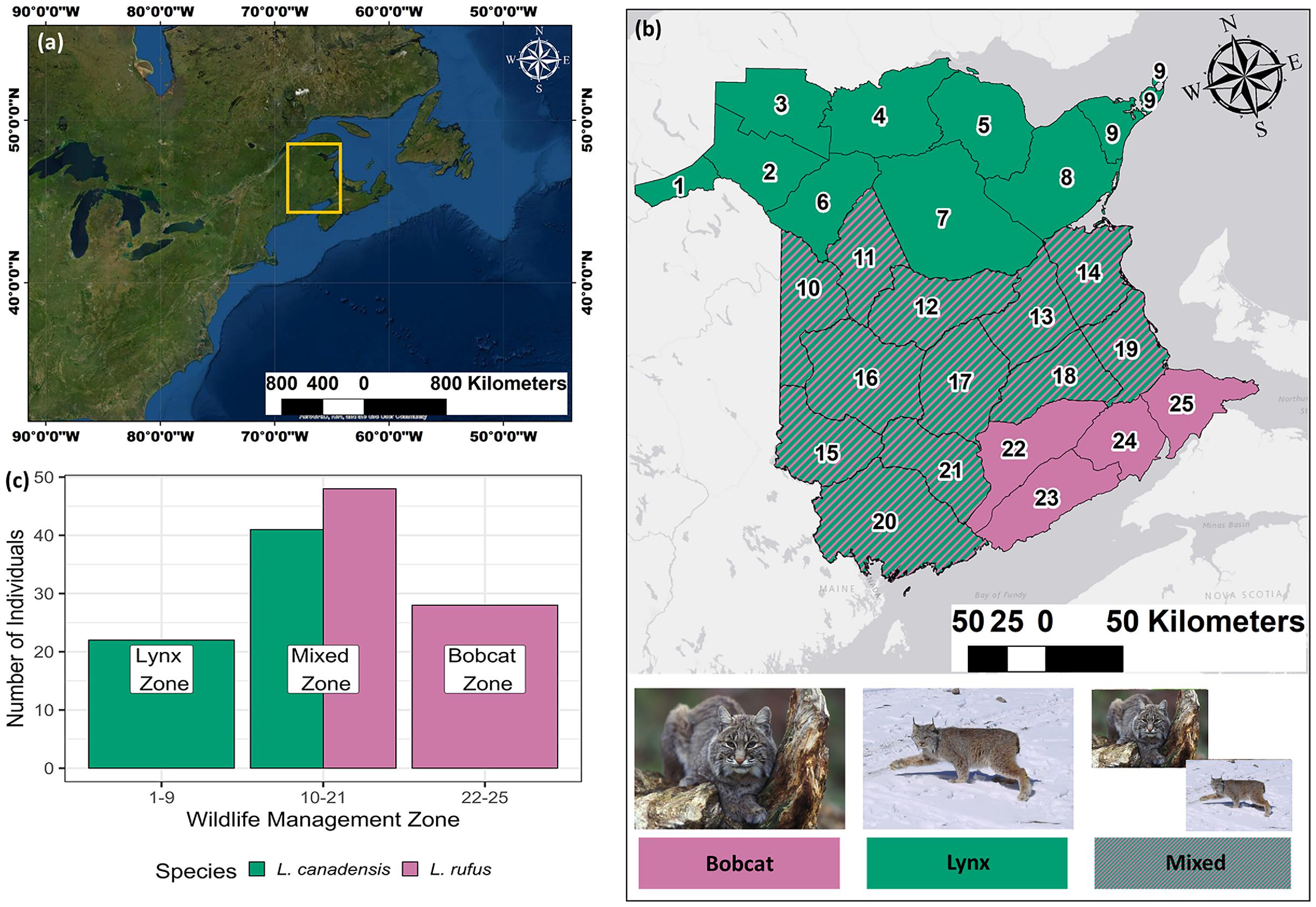
Bobcat and lynx collected for this study were unevenly distributed across a latitudinal gradient (
Fig. 1); lynx were the only species collected in the northernmost WMZs (hereafter, “Lynx zone”), whereas bobcats were the only species collected in the southernmost WMZs (hereafter, “Bobcat zone”). The center of the province included WMZs with both bobcat and lynx (hereafter, “Mixed zone”). Recorded masses for lynx were 9331 ± 1816 g (
n = 63), and those for bobcat (
sans pelt) were 7912 ± 2799 g (
n = 82). Males outnumbered females in both species (bobcat: 47 males and 35 females; lynx: 39 males and 26 females). The sex of one lynx could not be determined (
n = 1).
Samples of putative prey species were obtained from independent collections at the University of New Brunswick (G. Forbes, pers. Comm.). Prey items included snowshoe hares (n = 6), red squirrel, Tamiasciurus hudsonicus (Erxleben 1777) (n = 7), eastern chipmunk, Tamias striatus (Linnaeus, 1758) (n = 2), flying squirrel, Glaucomys sabrinus (Shaw, 1801) (n = 5), grey squirrel, Sciurus carolinensis (Gmelin 1788) (n = 1), red-backed vole, Myodes rutilus (Pallas 1779) (n = 6), and meadow vole, Microtus pennsylvanicus (Ord 1815) (n = 4). Putative prey samples were collected ad hoc from a variety of locations in NB between 2010 and 2020, and were frozen prior to analysis. The distribution of putative prey samples did not match that of bobcat and lynx; however, stable isotope analysis of other herbivorous mammals (white-tailed deer, Odocoileus virginianus (Zimmerman 1780), and moose, Alces alces (Linnaeus 1758)) sampled from each WMZ in New Brunswick indicates that there is little variability in terrestrial isotopic baselines throughout our study area (Table S1). All organisms sampled in this study were treated in accordance with University of New Brunswick animal care permits: #18026, #17032, and #06019.
Laboratory analysis
All wild cats were dissected at the DNRED laboratory. In total, post-mortem tissue samples were taken from 82 bobcat and 66 lynx. Samples (approx. 1 cm
3) of both liver and leg muscle were obtained from each specimen for stable isotope analysis. Leg muscle samples were also obtained from all putative prey samples. Samples were oven dried at 60 °C for 48 h, and subsequently ground to a homogenous powder. Subsamples (1 ± 0.1 mg) were weighed into aluminum foil capsules and submitted to the Stable Isotopes in Nature Laboratory at the University of New Brunswick (SINLAB) for carbon (δ
13C) and nitrogen (δ
15N) stable isotope analysis. At SINLAB, samples were combusted and analyzed in a Delta Plus continuous-flow isotope-ratio mass spectrometer (Thermo Finnigan GmbH, Bremen, Germany) connected to a Carlo Erba NC2500 elemental analyzer (ThermQuest S.p.A., Milan, Italy). Carbon and nitrogen isotope ratios are reported relative to Vienna PeeDee Belemnite and atmospheric nitrogen, respectively. Analytical precision, calculated as the standard deviation of repeat analyses of in-house standards, was estimated as 0.1 ‰ for both δ
13C and δ
15N: nicotinamide (δ
13C and δ
15N mean ± SD: −32.3 ± 0.03‰ and 2.0 ± 0.06‰), bovine liver (−18.8 ± 0.05‰ and 7.2 ± 0.02‰), muskellunge muscle (−22.3 ± 0.1‰ and 14 ± 0.1‰), and USGS 61 (USGS, Reston, VA, USA; −34.8 ± 0.08‰ and 2.9 ± 0.1‰). Liver δ
13C values from 2016 to 2017 were arithmetically corrected using a liver-specific model (
Logan et al. 2008). In 2017–18, lipids were chemically removed using repeated 2:1 chloroform:methanol rinses. No chemical or arithmetic lipid correction was performed on the muscle data as elemental C:N values were below 4, indicating that lipids were unlikely to have an effect on those data (
Post 2002).
Data analysis
All statistical analyses were completed in the computational language R and all graphs were produced using ggplot (
Wickham 2016;
R Core Team 2021). To avoid skewing our model estimates, we initially tested for and removed outliers in our isotope data using Grubb's test. We also excluded one lynx discovered in the bobcat zone and one bobcat taken from the lynx zone from our analysis. Both species are capable of dispersal over long distances, and we assumed these two individuals were not associated with an ecologically relevant coexisting population. Following outlier removal, PERMANOVA was implemented through the vegan package to test for differences in the bivariate mean (δ
13C and δ
15N) of bobcat and lynx, considering year, species, tissue, sex, and zone as interacting factors (
Oksanen et al. 2022). Evidence for an effect of year was absent in our PERMANOVA results, so we pooled data from 2016–17 and 2017–18 for subsequent comparisons. Furthermore, weak evidence for a significant effect of sex in our PERMANOVA was not supported by significant univariate differences between any groups for either isotope, so data for both sexes were pooled as well. One lynx was excluded from PERMANOVA as its sex was undetermined. Permutations were set to 999, and significance was assessed at
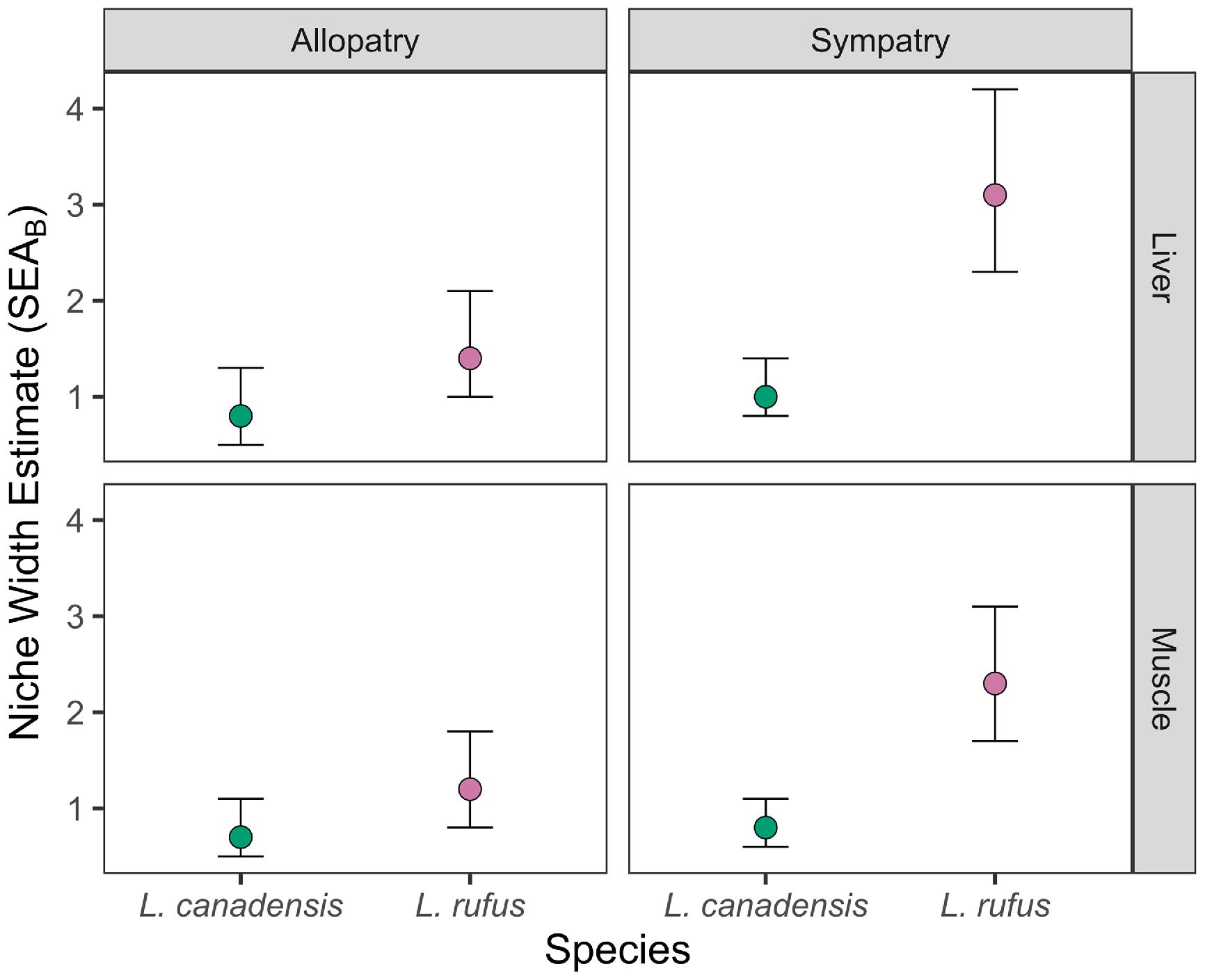
P < 0.01. For each year–species–tissue–zone combination, we calculated isotopic niche width as a standard ellipse area using δ
13C and δ
15N in the SIBER package (
Jackson et al. 2011). Bayesian standard ellipse area (SEA
b) was calculated for each species–zone–tissue combination, and two groups were considered to have a significantly different isotopic niche width when the 95% credible intervals of SEA
b did not overlap.
To examine differences in δ13C and δ15 N of liver and muscle tissues, we used non-parametric Welch t-tests to make pairwise comparisons among four groups: bobcat sampled in the bobcat zone (Bb), bobcat sampled in the mixed zone (Bm), lynx sampled in the mixed zone (Lm), and lynx sampled in the lynx zone (Ll). We first compared across species to contrast the resource use of bobcat and lynx in allopatry (Bb × Ll) and sympatry (Bm × Lm). We then determined whether the stable isotope composition of either species differed in the presence of the other species by comparing values within each species in sympatry and allopatry with its counterpart (i.e., Bb × Bm and Ll × Lm).
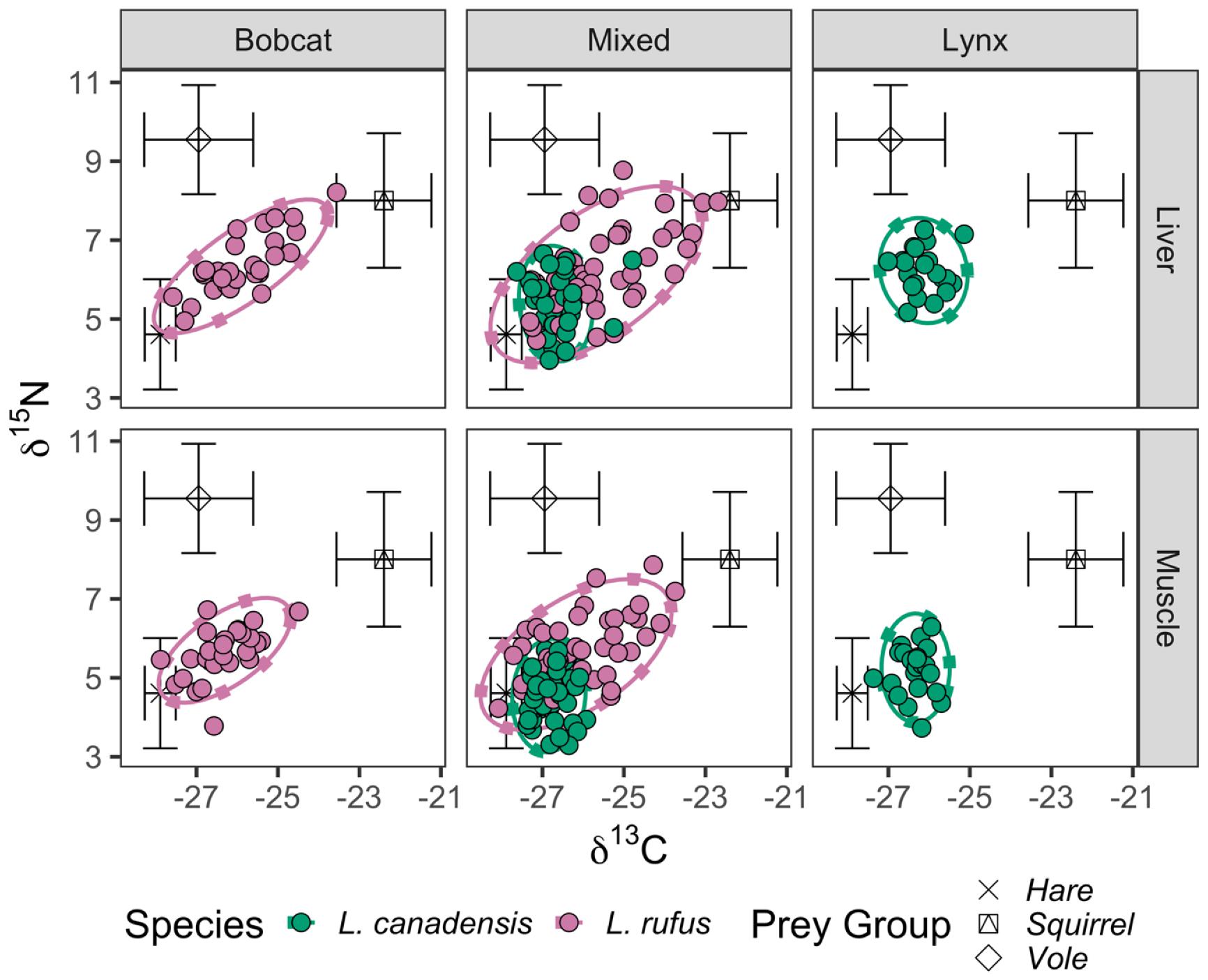
To assess individual specialization within each species, we used linear regression to examine the relationship between isotope ratios of muscle and liver tissues in individual bobcat and lynx. Separate analyses were performed for δ13C and δ15N stable isotope ratios, but data from both years was pooled. Linear models were also used to test for ontogenetic dietary shifts among groups by regressing stable isotope compositions on organismal mass.
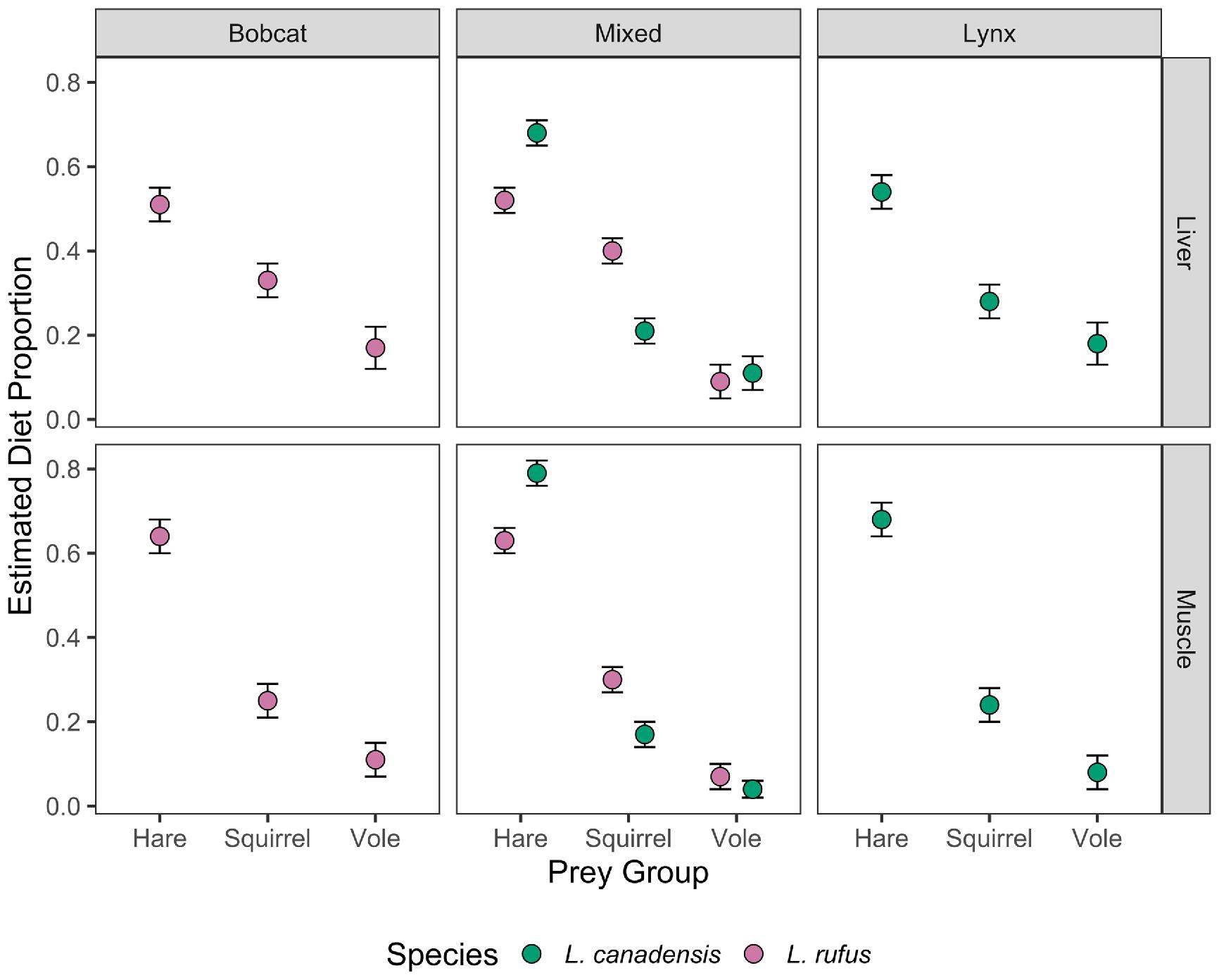
The stable isotope mixing model SIMMR was used to estimate the contributions of putative prey items to the diet of both bobcat and lynx (
Parnell et al. 2010). Prey were combined into three categories based on body size and behavior: hares (snowshoe hares), voles (meadow vole and red-backed vole), and squirrels (red squirrel, grey squirrel, flying squirrel, and eastern chipmunk). To assess variation in diet among species, zones, and time periods, separate models were run for each of the factor combinations outlined above. Diet-tissue offsets of 0.4‰ (±1.3) and 3.4‰ (±1) were used for δ
13C and δ
15N, respectively (
Post 2002). Notably, our mixing models assumed consumption of ungulates and avian species was negligible and that the diet-tissue offsets for liver and muscle in both bobcat and lynx were similar (
Tieszen et al. 1983;
Sponheimer et al. 2006). Two groups were considered to have significantly different resource use when the 95% credible intervals did not overlap.
We were interested in investigating the implications of the correlation between bobcat size and isotope composition in the mixed zone (see Results: Allopatric and sympatric comparison). To do this, we used regression equations between bobcat size and isotope composition to estimate δ13C (δ13Cestimate = (0.0001627899) * (Mass (g)) – 27.36888; P = 2.0 × 10−16) and δ15N (δ15Nestimate = (0.0001445833) * (Mass (g)) + 4.532383; P = 5.0 × 10−4) of bobcats similar in size to the largest (14 061 g) and smallest (2778 g) sampled in our study. We modeled the isotope composition of the large and small bobcat, rather than using the measured isotope composition of large and small cats directly, so that our model estimates would be consistent with the mean bobcat diet at different sizes and would be less skewed by individual variability among cats of differing sizes. We then estimated the diet of these modeled individuals using the same mixing model framework described in the preceding paragraph.
Discussion
Our study examined the ecology of bobcat and lynx at their range contact zone in southeastern Canada. Our data were consistent with previous work indicating that bobcat and lynx exhibit distinct generalist and specialist strategies, respectively (
Peers et al. 2012;
Gobin et al. 2022). Lynx throughout our study had a relatively narrow isotopic niche, and we determined there was a strong positive relationship between the stable isotope composition of lynx muscle and liver, indicating individual specialization was prevalent. In contrast, patterns of bobcat foraging were more complicated. Whereas lynx were snowshoe hare specialists throughout the study, bobcats foraged as specialists in allopatry and generalists when lynx were present. Overall, we found that the presence of a congeneric competitor drove both species to modify their niches, but at alternate ends of the generalist–specialist axis.
Our hypothesis was that sympatric bobcat and lynx compete for prey, which would result in niche displacement. This hypothesis was supported by data, as both species appeared to modify their trophic niche in sympatry. Lynx throughout our study constituted a relatively uniform population of individual hare specialists, whose degree of hare reliance increased when competing with bobcat. Our findings are consistent with previous work indicating snowshoe hares are the dominant prey of lynx in other parts of their range also (
Squires and Ruggiero 2007;
Ivan and Shenk 2016). Increased use of snowshoe hare by sympatric lynx provides evidence that the link between a specialist predator and their prey may be strengthened by interspecific competition (
Jones and Post 2016;
Halloway and Brown 2021). Depredation of alternative prey items or fine-scale habitat partitioning may have promoted increased predation on snowshoe hare by lynx when in sympatry with bobcats (
Murray and Boutin 1991;
Bunnell et al. 2006;
Morin et al. 2020).
In contrast, bobcats consumed less hare than lynx and expanded, rather than contracted, their isotopic niches in sympatry. Our estimates for consumption of hare by bobcats were similar to previous estimates from populations in Nova Scotia but greater than those for bobcats living in the United States (
Matlack and Evans 1992;
Newbury and Hodges 2018). Furthermore, correlations between the carbon and nitrogen stable isotope compositions of muscle and liver were stronger in bobcats from the mixed zone relative to bobcats from the bobcat zone, indicating that individual specialization was probably more prevalent in bobcats from the mixed zone. This result may indicate that the expansion of the sympatric bobcat's ecological niche at the population level was counterbalanced by decreased niche breadth at the individual level. This trade-off may help minimize intraspecific competition within bobcat populations also stressed by interspecific competition with lynx.
We initially described decreased consumption of alternative prey by lynx in sympatry with bobcats; it follows that lynx increased use of alternative prey when bobcats were absent. The niche-broadening observed is consistent with ecological release, as niche-broadening is thought to be driven by intraspecific competition when niche-narrowing interspecific competition is relaxed (
Araújo et al. 2011;
Jones and Post 2016). Nonetheless, our data lacked strong evidence for any specific form of ecological release (e.g., niche variation or parallel;
Bolnick et al. 2010). In contrast to lynx, the decreased niche breadth of bobcats in allopatry was inconsistent with our expectations from ecological release theory (
Bolnick et al. 2010).
Bobcat behavior is highly plastic, and they are known to alter their dietary habits based on prey availability (
Young et al. 2019;
Draper et al. 2022). Therefore, underlying differences in prey abundance could also be a potential driver of this phenomenon (
Araujo and Moura 2022). Another explanation for the contrasting responses of bobcat and lynx is that hare availability was not limiting for allopatric bobcats during our study. Therefore, specializing in snowshoe hare may have been the preferred strategy from a foraging efficiency perspective (
Svanbäck and Bolnick 2007). Moreover, our study also took place over a short time period and in a region characterized by ongoing changes to land-use and study-species abundance; a longer term study could yield different results.
We propose that the niche-diversifying effects of intraspecific competition might be dampened or overcome if selection of high-quality prey is strongly favored, as is the case when predator abundance is low or prey availability is high (
Bolnick et al. 2010). Specialization on hare is an efficient strategy for ambush predators like lynx because the energy spent on foraging is likely comparable across different prey species, and capturing one hare provides a similar amount of energy as multiple squirrels or voles (
Nellis et al. 1972;
Cumberland et al. 2001). Although consumption of alternative prey has not been correlated to long-term stress in the lynx, changes to foraging efficiency can drastically affect individual success (
Illius et al. 1995;
Burstahler et al. 2019). For bobcats, increased niche width in sympatry with competitors may help them get established in new habitats, whereas decreasing niche width to focus on high-energy prey when interspecific competition is relaxed promotes population growth.
The study of coexistence and character displacement usually focuses on adult traits (
Anaya‐Rojas et al. 2021). Our results provide evidence that niche displacement in bobcats may be ontogenetically structured, although we are not the first to determine ontogenetic changes in bobcat diet (
Fritts and Sealander 1978;
Litvaitis et al. 1984;
Rose 2015). Specifically, we determined that larger bobcats reduced hare consumption in sympatry with lynx. Although numerous factors contribute to an organism's foraging ecology, we presume competition with lynx played a role specifically because the observed diet-shift was not apparent in bobcat-zone bobcats. One explanation for the observed decrease in estimates of hare consumption by larger bobcats could be the introduction of ungulates, such as white-tail deer, into their diet. Most depredation of white-tailed deer in New Brunswick is by coyote, but bobcat have been recorded to kill adult deer during winters and neonates in mid-summer (
Whitlaw et al. 1998;
Ballard et al. 1999). Unfortunately, we were unable to conclusively differentiate increased consumption of deer from increased consumption of voles and squirrels.
Our interpretations rest on a variety of assumptions related to bobcat and lynx behavior, as well as the relationship of stable isotope measurements to their prey selection. A critical assumption was that the migration of wild cats among our zones was an insignificant source of variability in our data. We feel this assumption is reasonable since both species tend to establish stable home ranges; however, we acknowledge that both bobcat and lynx are capable of dispersing over significant distances (
Poole 1997;
Johnson et al. 2010). Future studies could benefit from improved resolution of the abundances and dispersal characteristics of bobcat and lynx within the study area. Although we inferred that competition among bobcat and lynx played a role in shaping the patterns we observed, competitive interactions are complex, and accurately characterizing them using spatiotemporally limited data like ours is impossible. More robust methods of inferring competition include experimental manipulations and integrating data from much broader spatiotemporal scales. Unfortunately, collecting and aggregating broad-scale data are often onerous, and experimentally manipulating the relative abundance of wide-ranging carnivores, while controlling for other variables like prey abundance, is not always feasible. To this point, while results from our survey of bobcat and lynx in New Brunswick suggest an interesting competitive interplay, we cannot determine how generalizable these responses are or discount that unmeasured environmental gradients were amplifying or dampening the observed patterns. We suggest that future studies incorporate measurement and analysis of environmental covariates to more robustly constrain what proportion of observed changes are attributable to competitive interactions.
Anthropogenic activities are driving rapid change in global environmental conditions and species distributions (
Laliberte and Ripple 2004;
Manlick and Pauli 2020). Periods of rapid environmental change have the potential to be punctuated by rapid ecological change (
Walther et al. 2002). Accurately forecasting changes to ecological communities and determining appropriate management actions to discourage undesirable changes (e.g., extirpation of lynx) requires an understanding of their interactions with the environment and other species. We focused on the interaction of bobcat and lynx, and conclude that conservation of lynx should continue to involve maintenance of snowshoe hare habitat. Prey-switching could work to mitigate lynx population collapse during periods of low hare abundance, but our results indicate that both hares and alternative prey for lynx could become less available due to the activity of bobcats (
Peers et al. 2014). Therefore, as bobcat abundances increase, the availability of snowshoe hares may become more critical for lynx (
Popescu et al. 2021).