Introduction
Freshwater ecosystems cover less than 1% of the earth’s surface and are inhabited by approximately 40% of global fish biodiversity, but represent some of the most threatened habitats in the world (
Dudgeon et al. 2006;
Göthe et al. 2015). Habitat loss and degradation, over exploitation, pollution, and flow modification represent the greatest threats to freshwater ecosystems, all of which are directly associated with anthropogenic activities and development (
Dudgeon et al. 2006;
Arthington et al. 2016;
Reid et al. 2019). The deterioration and destruction of freshwater habitats highlight why freshwater fishes are some of the most imperiled organisms in the world (
Duncan and Lockwood 2001;
Jelks et al. 2008).
Although up to 30% of freshwater fishes are threatened with extinction (
Collen et al. 2014;
Miranda et al. 2022), not all trophic guilds are equally vulnerable to habitat degradation. Elevated sediment input is likely the single most common aspect of degraded stream habitat (
Kemp et al. 2011); consequently, the guild of benthic insectivores are particularly vulnerable to watershed development, because their habitat and prey resources are directly impacted by sediment deposition (
Rabeni and Smale 1995;
Walters et al. 2003). This vulnerability is compounded for riffle-dependent benthic insectivores with high water velocity requirements (
Aadland 1993), since flow reduction is another frequent consequence of human development of stream ecosystems (
de Graaf et al. 2019). Additional risk exists if the distribution of a benthic insectivore coincides with human development pressure, and if they have small body size, since small-bodied species are also more susceptible to extinction (
Reynolds et al. 2005;
Vélez-Espino and Koops 2012;
van der Lee and Koops 2015).
The Nooksack Dace (
Rhinichthys cataractae sp
. cataractae), a small, benthic cyprinid and riffle-specialist insectivore, is a genetically and morphologically distinct form of Longnose Dace (
Rhinichthys cataractae) of uncertain taxonomic status (
COSEWIC 2007). It diverged from other forms of
R. cataractae during geographic isolation in a glacial refuge in Washington State during the Pleistocene (
Taylor et al. 2015). Nooksack Dace is endemic to only four watersheds in Canada in the lower Fraser Valley (LFV) of British Columbia (
Pearson et al. 2007), where it is listed as Endangered under the federal Species at Risk Act (SARA;
Fisheries and Oceans Canada 2020) and by the Committee on the Status of Endangered Wildlife in Canada (
COSEWIC 2018). Rapidly declining populations are attributed to a range of impacts including habitat loss and degradation, effects which are compounded by low flows and deteriorating water quality, all resulting from anthropogenic development within their watersheds (
Pearson 1998,
1999;
Pearson et al. 2007;
Boyd et al. 2022).
Dace are typically found in riffle habitat (
Pearson et al. 2007), preferring shallow moving water (>0.25 m·s
−1) (
McPhail 1996) over coarse unembedded substrate. Individuals will forage in pools during drought when riffles dry (
Avery-Gomm et al. 2014) and as young-of-the-year when they inhabit shallow, marginal pools with fine substrate adjacent to riffle spawning habitat (
McPhail 1996;
Pearson 1998,
1999). Dace rely on the interstitial spaces amongst the coarse substrate particles of the bed for refuge from flow and predation, for spawning, and for feeding (
Pearson 1999). However, their dependence on riffles with unembedded coarse substrate leaves the species particularly vulnerable to habitat degradation resulting in the deposition of fine sediment.
Similar to many valley bottom floodplain landscapes throughout the world, the LFV has been transformed by human activities over the last 200 years. Here, a mix of temperate rainforest and wetlands became a landscape dominated by agriculture, resource extraction, and increasing urbanization (
Boyle et al. 1997;
Pearson 1998). In the process, 15% of the streams within the LFV no longer exist, were paved over, or piped (
Fisheries and Oceans Canada 1998) with many more channelized and dredged (
Pearson et al. 2007). While channelization is beneficial for drainage and flood mitigation, it results in homogenous habitat lacking the physical complexity associated with a natural meandering channel (
Rambaud et al. 2009;
Lennox III and Rasmussen 2016). This habitat complexity is essential to accommodate the diversity of habitats required by different life history stages for spawning, juvenile rearing, feeding, and hydraulic refuge during floods (
Pearson et al. 2007).
In addition to direct habitat simplification through channelization, alteration of upslope processes by agriculture and urbanization typically increase fine sediment inputs to streams (
Kemp et al. 2011). Although the transport and storage of organic and inorganic sediments is an integral process driving substrate heterogeneity and nutrient transport, excessive deposition of fine sediment alters channel structure and substrate properties (i.e., particle size, interstitial volume, and hyporheic flow) in ways that are often detrimental to fish and invertebrate production (
Wood and Armitage 1997;
Florsheim et al. 2008;
Scholl et al. 2022). Urban storm run-off, agricultural effluent, and accelerated bank erosion all increase the amount and frequency of sediment inputs (
Suttle et al. 2004).
Infilling of interstitial space by fine sediment inputs directly removes foraging and refuge habitat for benthic fish like Nooksack Dace, but can also limit the interstitial flow of oxygen rich water to developing eggs and larvae (
Pearson et al. 2007;
Kemp et al. 2011;
Kawanishi et al. 2015). Excessive fines can also decrease benthic invertebrate diversity and production by clogging substrate interstices (
Bo et al. 2007;
Burdon et al. 2013), an effect that is particularly pronounced in small lowland streams that lack the hydraulic power to export deposited fines (
Naden et al. 2016). The unembedded substrate of riffle habitats represent hotspots of benthic invertebrate production in streams (
Poff and Huryn 1998;
Scholl et al. 2022). Infilling of interstitial space can greatly diminish prey abundance for fish (
Suttle et al. 2004), in addition to reducing space available for refuging (
Finstad et al. 2007), providing both direct and indirect pathways for interstitial accumulation of fine sediment to impact fish.
Riffle habitat restoration is a practical approach to recover endangered riffle specialists, but its effectiveness and underlying pathways of effect are rarely rigorously tested. To assess the effectiveness of riffle habitat restoration at increasing abundance of a typical riffle benthivore, we added coarse unembedded gravel and cobble substrate to existing riffles in two streams with contrasting riffle habitat quality: one with unembedded substrate and high dace densities, and a second with scarce coarse substrate and few dace. The main objectives were to evaluate the overall effectiveness of coarse substrate addition at increasing dace abundance, and to identify the specific processes and pathways that lead to increased density. Our expectations were that any change in dace abundance following substrate addition would be most pronounced in the stream with more degraded riffles, and minimal in the stream with abundant coarse substrate. We executed a before-after-control-impact (BACI;
Green 1979) experiment to determine the outcome of gravel and cobble additions to habitat, invertebrates, and fish in two representative Nooksack River tributaries, as described below. We specifically hypothesized that dace abundance would be correlated with the volume of available interstitial space, which would be positively associated with mean particle size and would increase with substrate addition. We also predicted that benthic invertebrate biomass would increase with the availability of interstitial spaces, and would correlate with observed dace density. Finally, we expected a positive response of Nooksack Dace to coarse substrate addition both in terms of greater adult density and increased spawning success (measured in terms of more juvenile recruits following substrate addition).
Materials and methods
Study site and species
Experiments were performed in Bertrand and Pepin Creeks, two transboundary tributaries of the Nooksack River that flow south from British Columbia into Washington State, United States (Fig. S1). Substrate was added to riffles distributed over approximately 1 km of stream channel in both systems (Tables S1 and S2). Bertrand Creek and Pepin Creek both have gradients of approximately 1%–2% and summer low flow wetted widths of approximately 3 m. The main habitat contrast between streams is that Bertrand Creek has a higher proportion of unembedded cobble substrate and abundant dace (1.4 dace·m
−2 in pre-treatment sampling), while cobble substrate is scarce and embedded in Pepin Creek, and dace density is low (0.2 dace·m
−2 in pre-treatment sampling). Bertrand Creek experiences higher peak flows than Pepin Creek (42 m
3·s
−1 vs. 1.5 m
3·s
−1, respectively;
Pearson 2004;
U.S. Geological Survey 2023) which creates a more mobile channel that more effectively exports fine sediment than the more stable Pepin Creek. However, Bertrand Creek also experiences lower summer minimum flows (0.02 m
3·s
−1 vs. 0.07 m
3·s
−1, respectively) because Pepin Creek has a greater groundwater influence (
Berg and Allen 2007).
Study design
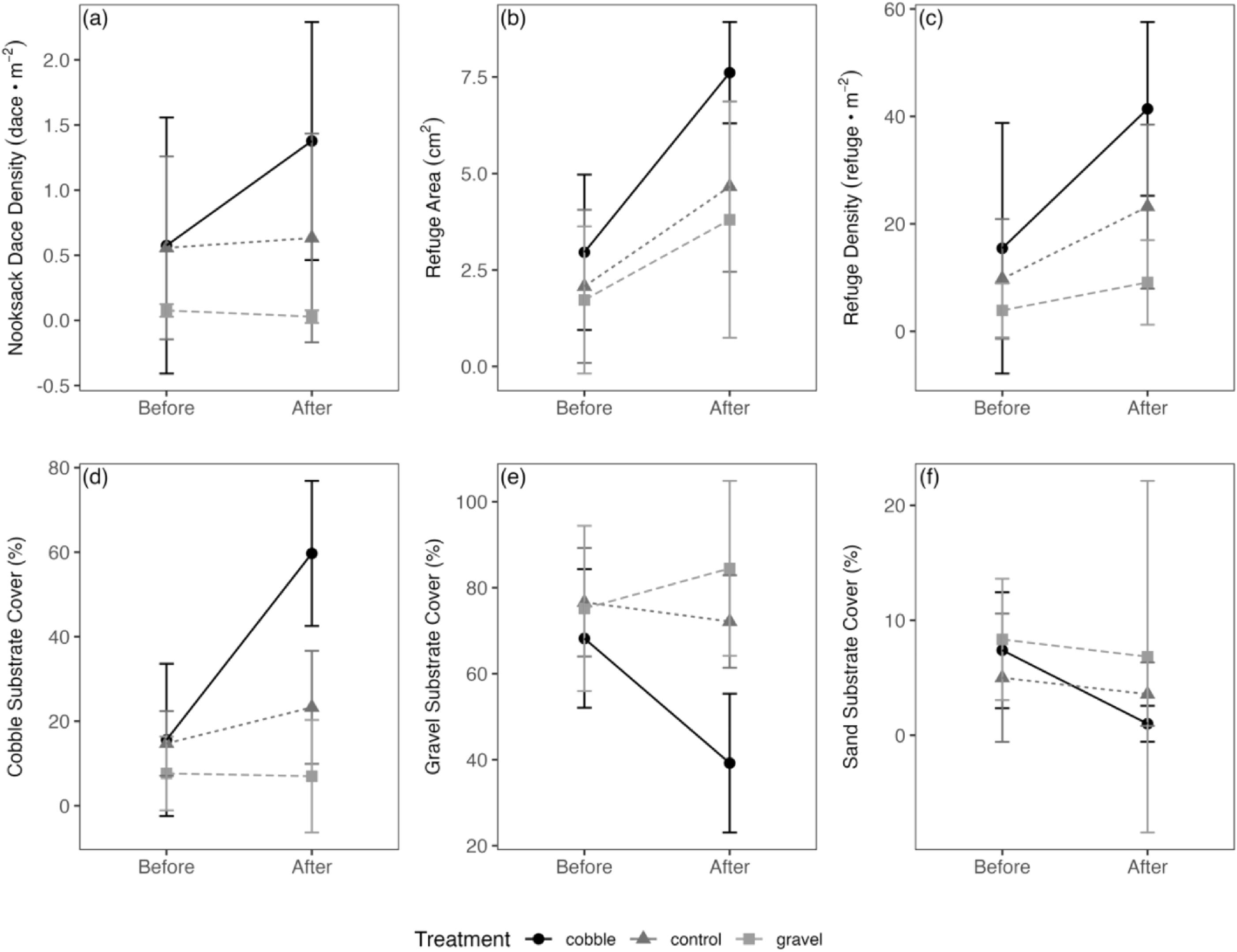
The substrate composition of control riffles was typically coarse gravel and cobble substrate embedded with accumulated fine substrate and sand (
Fig. 1a), but differed between streams. In Pepin Creek, control riffles tended to contain less cobble substrate cover and smaller particles that were more embedded. In comparison, control riffles in Bertrand Creek tended to contain more cobble substrate which tended to be less embedded, likely due to higher peak flows.
Restoration experiments included four treatments: control riffles that were unmanipulated, riffles with cobble substrate addition, riffles with gravel substrate addition, and riffles with both cobble and gravel addition as discrete adjacent patches; replicate substrate patches were 3 m long, with the exception of the combined cobble and gravel treatments that were 6 m long and included two sequential patches of gravel and cobble placed in longer riffles. This treatment was intended to assess whether close proximity to unembedded gravel substrate with potentially high benthic invertebrate abundance would increase Nooksack Dace density in adjacent cobble substrate. More treatments were placed in Pepin Creek where lower dace abundance and poorer substrate quality was expected to elicit a larger and more informative response to riffle restoration than in Bertrand Creek, where dace and cobble were already abundant. However, the number and location of replicate riffles was ultimately limited by resources and site access. Three replicates of each of the gravel, cobble, combined cobble-gravel, and control treatments were installed in Pepin Creek (n = 12). However, while all four treatments were installed in Pepin Creek, site and resource constraints limited treatments in Bertrand Creek to only four cobble and four control replicates (n = 8). The assignment of treatments to riffles was randomized to the extent possible however, the location of some treatment replicates was partially constrained by riffle length and site access (i.e., some controls were assigned to riffles where substrate could not be added due to poor machine access). Because substrate-specific dace density in the treatment with adjacent cobble-gravel patches did not differ from density in the isolated gravel and cobble treatments, the combined treatment was merged with the gravel and cobble addition treatment replicates in post-restoration monitoring and assessment to simplify data analysis. Thus, post-restoration analysis included three control replicates and six replicates of both the gravel and cobble treatments in Pepin Creek (n = 15), and four replicates of control and cobble treatments in Bertrand Creek (n = 8).
The experiment was designed as a BACI study (
Green 1979), with sampling in all riffle replicates taking place in June to August 2021 prior to riffle restoration, and in June to August 2022, one year post-restoration. Response variables collected before and after substrate addition included substrate characteristics (i.e., particle size, percentage cover in different substrate classes, and interstitial volume) as well as benthic invertebrate and fish density, as described below.
Substrate was added to riffles in August 2021 during the seasonal low flow instream work window, after fish were removed by multi-pass electrofishing prior to habitat manipulation. Both gravel (2–5 cm
b-axis length) and cobble (8–30 cm
b-axis length) were added to riffles on top of existing substrate in uniform patches measuring approximately 4 m in length and spanning the approximately 3 m wetted width of the creek, for a total surface area of approximately 12 m
2 per riffle. Because both streams were small (approximately three meter wetted width at low flow) this also covered the entire length of many riffles (
Fig. 1b).
Substrate was added using a track excavator where possible, or by hand when access to the creek was limited by a full riparian buffer. Cobble substrate patches were established by adding a layer of smaller cobbles (i.e., 8–15 cm b-axis length) over the surface of existing riffle substrate, and then placing larger cobble (i.e., 16–30 cm b-axis length) on top to a total depth of 12–15 cm. Gravel patches were established through addition of a uniform layer of gravel substrate to a depth of approximately 6–10 cm. Record fall floods in 2021 post-restoration settled (re-arranged) substrate in a more natural configuration with minimal export of substrate beyond each treatment patch.
Measuring riffle habitat features
Surface substrate composition of riffles was measured before (July to August 2021) and after (July to August 2022) habitat restoration using modified Wolman Pebble Counts (
Wolman 1954). Particle diameter was measured at 25 locations on each of four 40 by 40 cm grids systematically placed along transects across each riffle. Each grid consisted of a 40 by 40 cm polyvinyl chloride pipe frame divided with string into 36 equal-area squares, with substrate particles selected at grid intersections.
Substrate particles were measured along the
b-axis (
Clapcott et al. 2011) and categorized into five size classes based on a modified Wentworth scale; fines (<0.25 mm), sand (0.25–2 mm), gravel (2–64 mm), cobble (64–256 mm), and boulders (>256 mm;
Wentworth 1922). Substrate was classified as fines or sand only when it fully buried underlying substrate (i.e., a fine layer of silt overlying a substrate of larger size was classified as the larger particle). Percent substrate cover in each riffle was estimated as the percentage of 100 measured particles in each substrate class.
Size and abundance of interstitial refuges was also measured within each of the four quadrats using short lengths of clear plastic tubing inserted into substrate interstices, as described by
Finstad et al. (2007). Tubing with diameters of 0.7, 1, 1.9, and 2.2 cm (Fig. S2) were used to probe and count interstices with a minimum length of 3 cm or greater, measured using the largest diameter tube that would fit within each potential refuge. The area of each identified interstitial refuge was then estimated by multiplying the diameter of the refuge by its length.
Fish and benthic invertebrate collection
Fish were collected from all riffles (i.e., control and treatment riffles), pre- and post-restoration by triple-pass, depletion electrofishing in August of 2021 and 2022, respectively (2021 and 2022 Scientific Fish Collection Permits SU21-624949 and SU22-718288). To prevent the escape of fish from the sample area, fish-tight stop nets were carefully installed across the wetted width of the channel at the upstream and downstream ends of riffles before fish collection. Capture effort (i.e., time spent electroshocking) was held constant for each pass in a single riffle. A conservative estimate of fish abundance in each riffle was calculated as the sum of the fish caught in all three passes, rather than a depletion estimate because low or inconsistent catches in Pepin Creek riffles precluded meaningful depletion estimates; density was estimated as total abundance divided by the surface area of the riffle. Capture efficiency of Nooksack Dace by electrofishing is in the range of 25%–35% (
Bonamis 2011;
Avery-Gomm et al. 2014), so that estimates of density from electrofishing could be rescaled to approximate true densities by multiplying by a factor of 3.3. However, rescaling Nooksack Dace density estimates would not have changed the relative treatment effects and so abundance estimates were left unexpanded. A minimum of 10 min separated the end of one electrofishing pass and the commencement of the next to allow the recovery of fish that escaped collection. Collected fish were sedated with MS2-22, identified, and measured for weight, and total length. Fish were placed in flow-through bins to recover prior to their release at the site of capture. Sampling procedures involving the collection of Endangered Nooksack Dace were designed and implemented in accordance with applicable laws (Fisheries and Oceans Canada Species at Risk Permits: 21-HPAC-00744 and 21-HPAC-00746) and approved by the Animal Care Committee at the University of British Columbia (permit: A21-0086) and followed Canadian Council for Animal Care guidelines.
Samples of benthic invertebrates were also collected from all replicate riffles in Pepin and Bertrand Creeks before and after restoration. A 15 cm wide Surber sampler (0.023 m2 sampling area) with 250 μm mesh was used to collect four benthic samples from each riffle in late July of 2021 and 2022, approximately two weeks prior to fish collection. Each sample was collected from a randomly chosen point at either 25%, 50%, or 75% across each of four evenly spaced transects across each riffle.
Samples were collected by disturbing the substrate by hand to a depth of 5 cm to dislodge benthic invertebrates into the downstream Surber sampler. Each sample was filtered through a 250 μm mesh sieve and preserved in 70% ethanol. Invertebrates were subsequently sorted from debris in the laboratory at 16 times magnification using a stereomicroscope and identified to family or subfamily according to
Merritt and Cummins (1996). The length of each invertebrate was measured by digitizing images projected from the microscope onto an adjacent digitizing pad using a drawing tube (
Roff and Hopcroft 1986). The dry weight biomass of each taxa was then estimated using published length–weight regressions for aquatic and terrestrial invertebrates (
Benke et al. 1999;
Sabo et al. 2002). To estimate the available biomass of prey for—dace, benthic invertebrate taxa were considered potential prey if they were previously documented in the diet of Nooksack Dace or considered vulnerable to predation, after
Avery-Gomm et al. (2014) and
McPhail (1996).
Data analysis
We performed two general analyses to understand the response of Nooksack Dace to both natural and experimental variation in substrate composition. First, we evaluated the relationships between substrate variables and dace density using univariate or multiple regression. Second, we assessed the response of both habitat and dace density to riffle restoration as a BACI experiment, testing for an interaction between time (before or after restoration) and treatment as the diagnostic for a significant restoration effect. All analyses were completed using R version 4.1.1 (
R Core Team 2022).
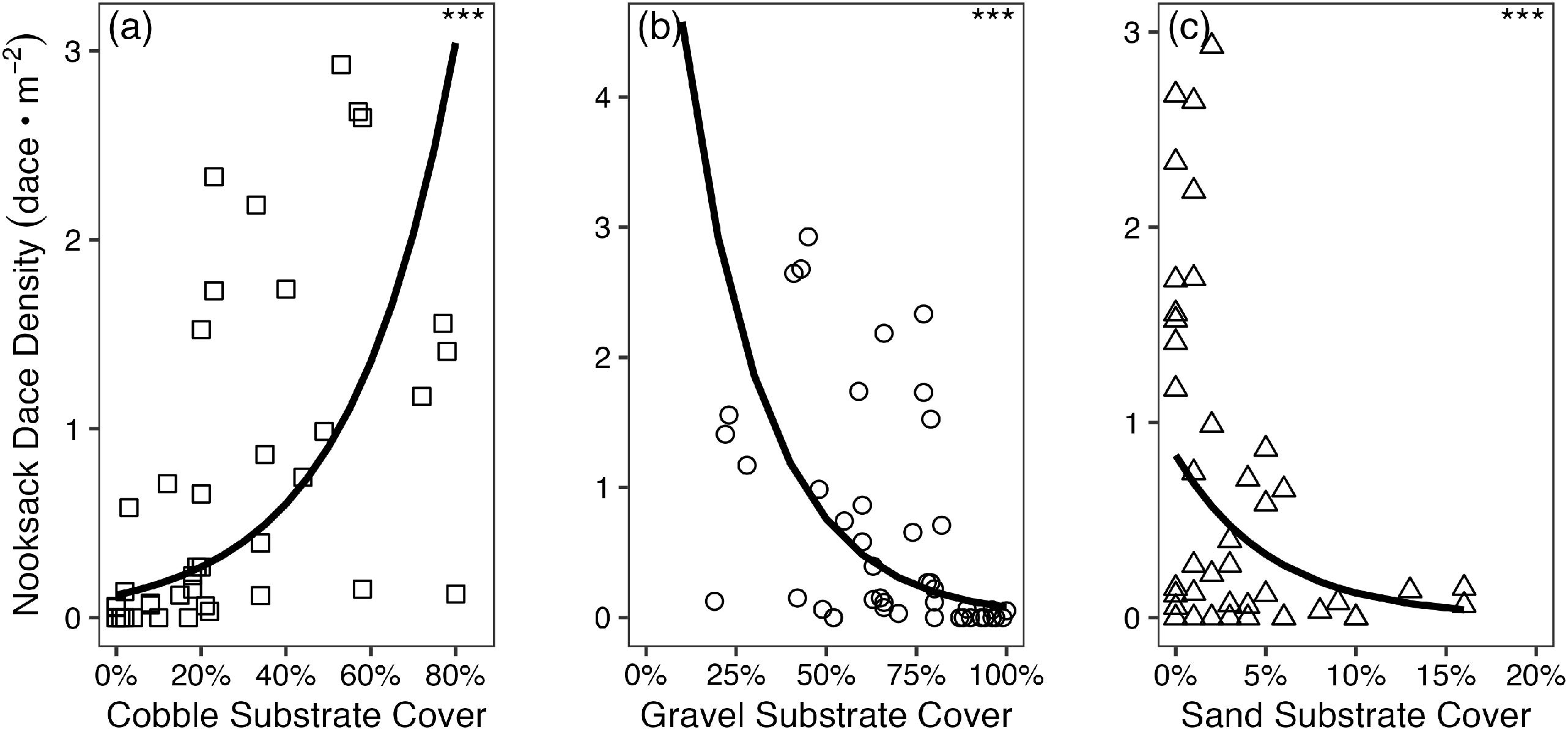
Using the regression approach, the influence of substrate attributes on dace density (dace·m
−2) across all riffles combined were evaluated as fixed effects in separate generalized linear mixed models (GLMM). To meet model assumptions, a Tweedie distribution with a log link function was used (Tweedie package;
Dunn and Smyth 2005). Stream system (Bertrand Creek vs. Pepin Creek) and restoration site (i.e., treatment replicates) were included as nested, random effects. The effects of substrate attributes on Nooksack Dace density were evaluated as separate univariate GLMM due to small sample size where the inclusion of additional predictor variables could reduce the statistical power of the model. Model fit was assessed using Q–Q plots of (DHARMa package;
Hartig 2022) and by verifying deviations from the uniformity of randomized quantile residuals (Kolmogorov–Smirnov test; performance package;
Lüdecke et al. 2021).
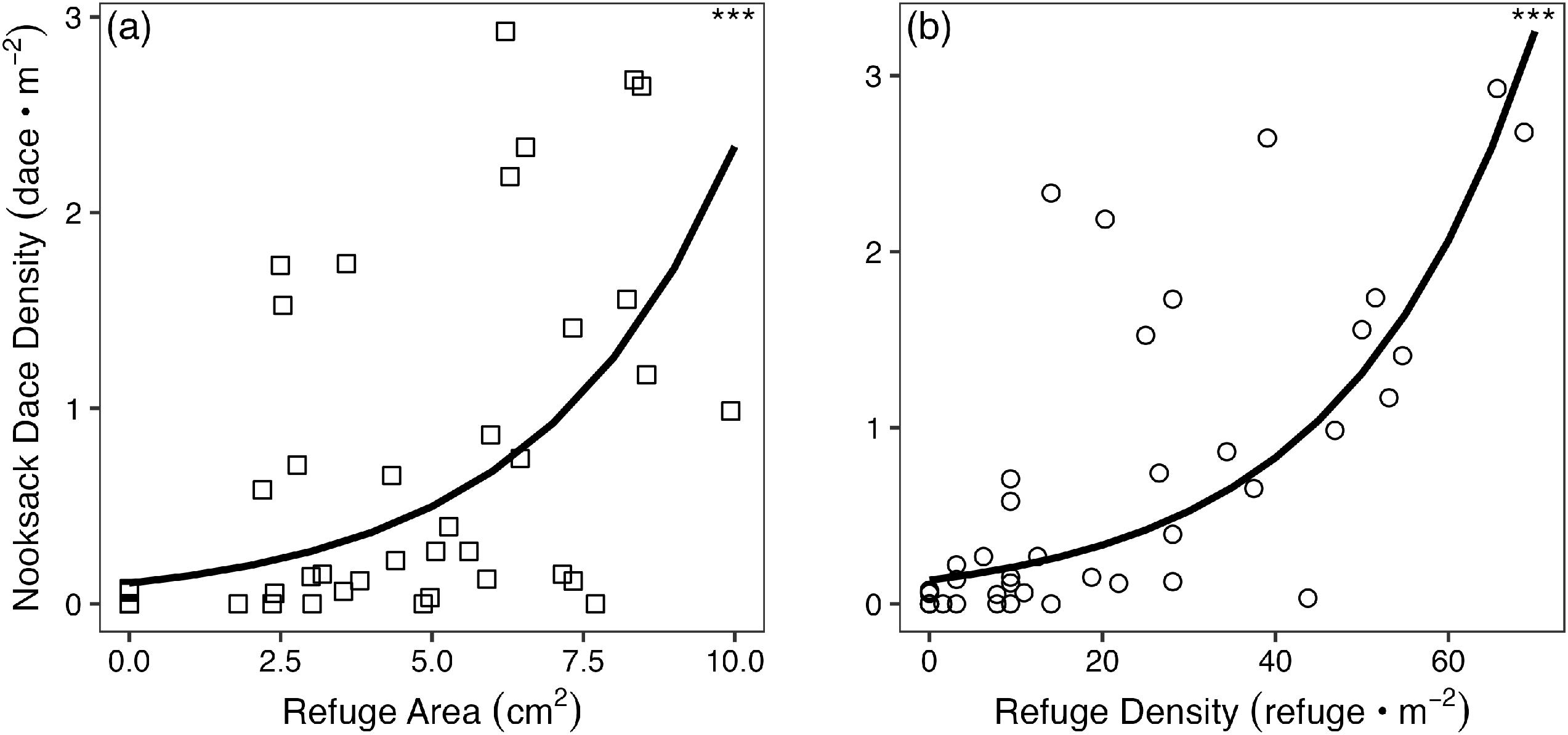
The density of interstitial refuges within a riffle was calculated as the number of identified refuges in a quadrat divided by quadrat area (0.16 m
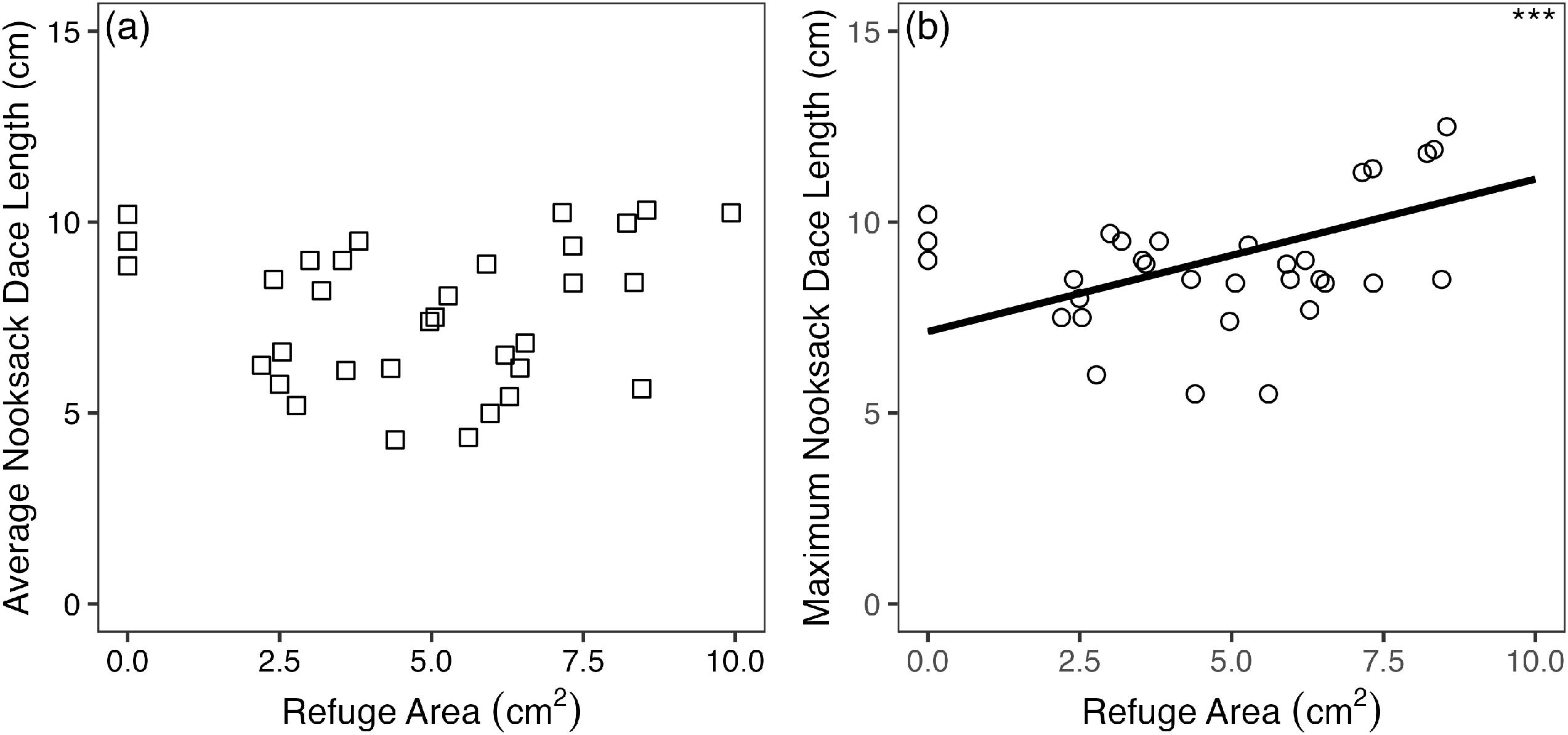
2). Area (two-dimensional footprint) of individual interstitial refuges was calculated as the product of average refuge length and diameter for each of the four diameter classes. The response of average and maximum dace total length to average interstitial refuge area (all diameter classes combined) was modelled using separate linear mixed models (LMM), with stream system and restoration site included as nested, random effects. Residuals were tested for normality and homogeneity using Shapiro–Wilks and Breusch–Pagan tests, respectively (
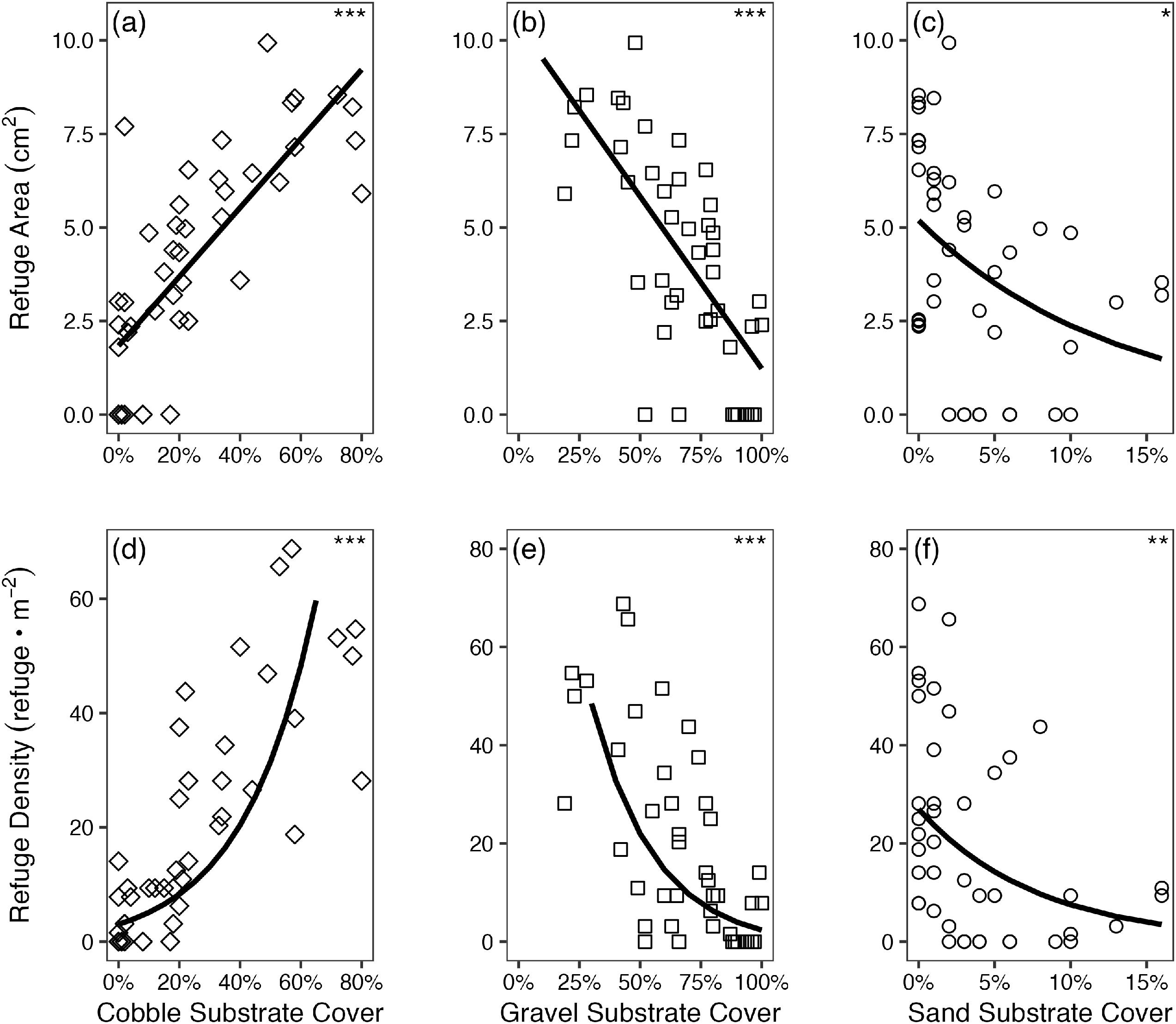
R Core Team 2022). The effect of cobble and gravel substrate cover on the density and area of interstitial refuges was also modelled using separate LMM. To meet model assumptions of homogeneity, response variables were log transformed when modeling the effects on cobble and gravel substrate cover on the density of interstitial refuges. The effect of sand substrate cover on the average density and area of interstitial refuges was modelled using separate GLMM, as described previously.
In addition to direct modelling of habitat and substrate effects on dace density as described above, we applied also analyzed the BACI design using a LMM to evaluate the effects of the riffle restoration intervention on substrate composition, interstitial refuge area and density, and Nooksack Dace density (
Roni et al. 2018). The diagnostic for a significant treatment effect was an interaction between time (two levels: before and after restoration) and restoration treatment (cobble, gravel, or control), with time and treatment as fixed effects and stream and restoration site treated as nested, random effects in the LMM. To normalize model residuals, response variables were square-root transformed for all LMMs with the exception of interstitial refugia area, which remained untransformed, and sand and gravel substrate cover, where the dependent variables were log transformed. Normality and homogeneity of residuals were tested using Shapiro–Wilk and Breusch–Pagan tests, respectively (
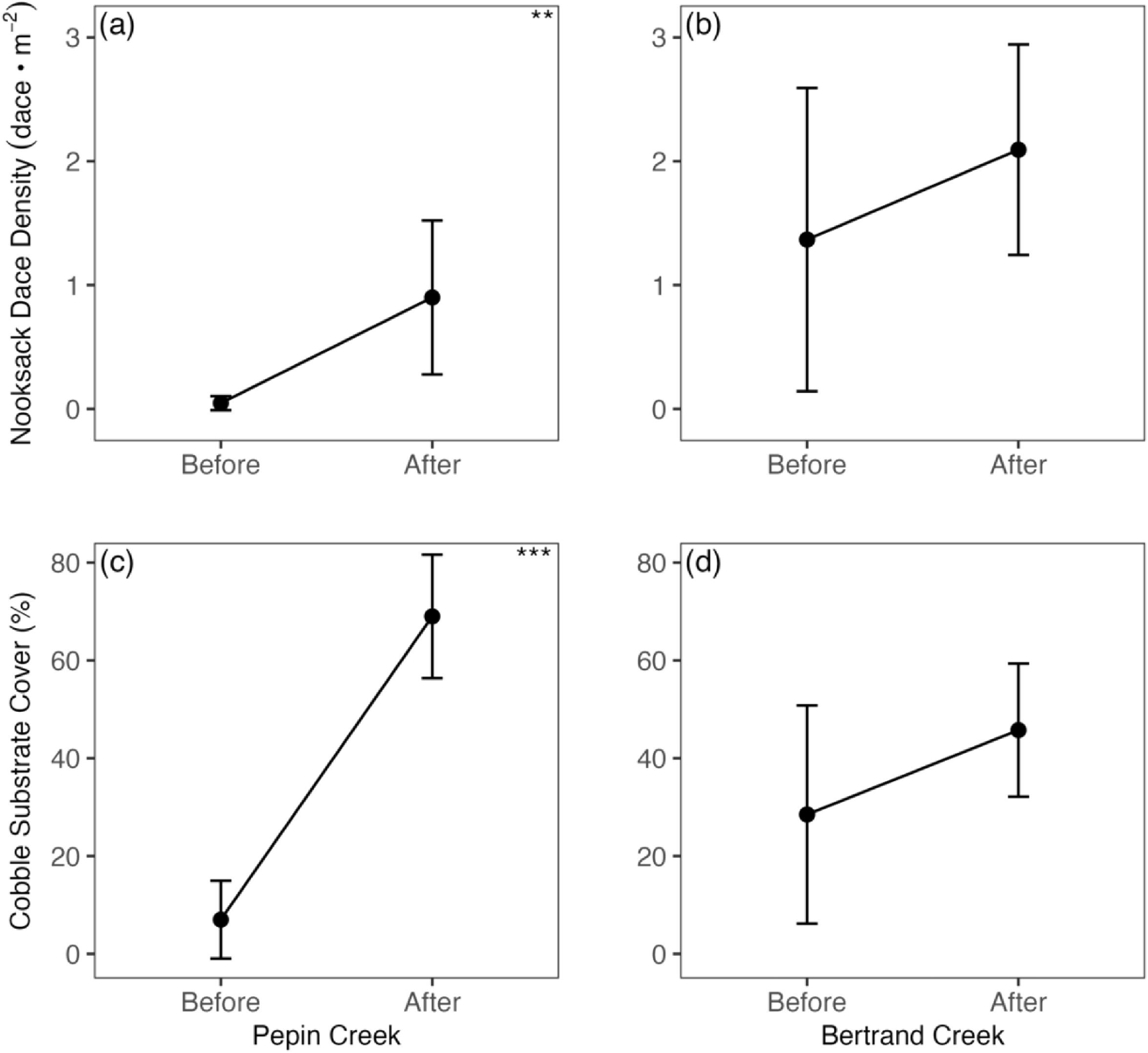
R Core Team 2022). Lastly, Wilcoxon Rank Sum tests were used to separately analyze changes in overall dace density and cobble substrate cover in Bertrand Creek and Pepin Creek. Wilcoxon Rank Sum tests were used because transformations could not normalize residuals.
The effects of substrate composition and interstitial refuges on benthic invertebrate biomass were assessed using separate LMM, as described above, with log transformations of invertebrate biomass to normalize residuals. To test for a positive effect of invertebrate biomass on dace density, GLMM was used, with stream system and restoration site treated as nested, random effects. A Wilcoxon Rank Sum test was used to compare invertebrate biomass between the two study streams. Lastly, a BACI design was used to examine whether riffle restoration had an effect on invertebrate biomass using LMM, as described above.
Discussion
The restoration of degraded habitat has become widespread in freshwaters and is a key element in the recovery of many species at risk (
Tear et al. 1993;
Follstad Shah et al. 2007;
Reid et al. 2016;
Gawecka and Bascompte 2021). However, the effectiveness of habitat restoration is rarely assessed (
Bernhardt and Palmer 2011), despite evaluation being an essential step in adaptive management. In this study, we examined whether Nooksack Dace density increased in response to riffle habitat restoration to assess whether it represents an effective recovery strategy for this Endangered species. We explicitly tested two general hypotheses for the potential role of coarse cobble substrate in supporting higher dace abundance: (i) that substrate interstices would provide increased habitat for benthic invertebrates, which increases prey abundance and therefore densities of benthic insectivores like Nooksack Dace and (ii) that interstices directly provide physical habitat for dace.
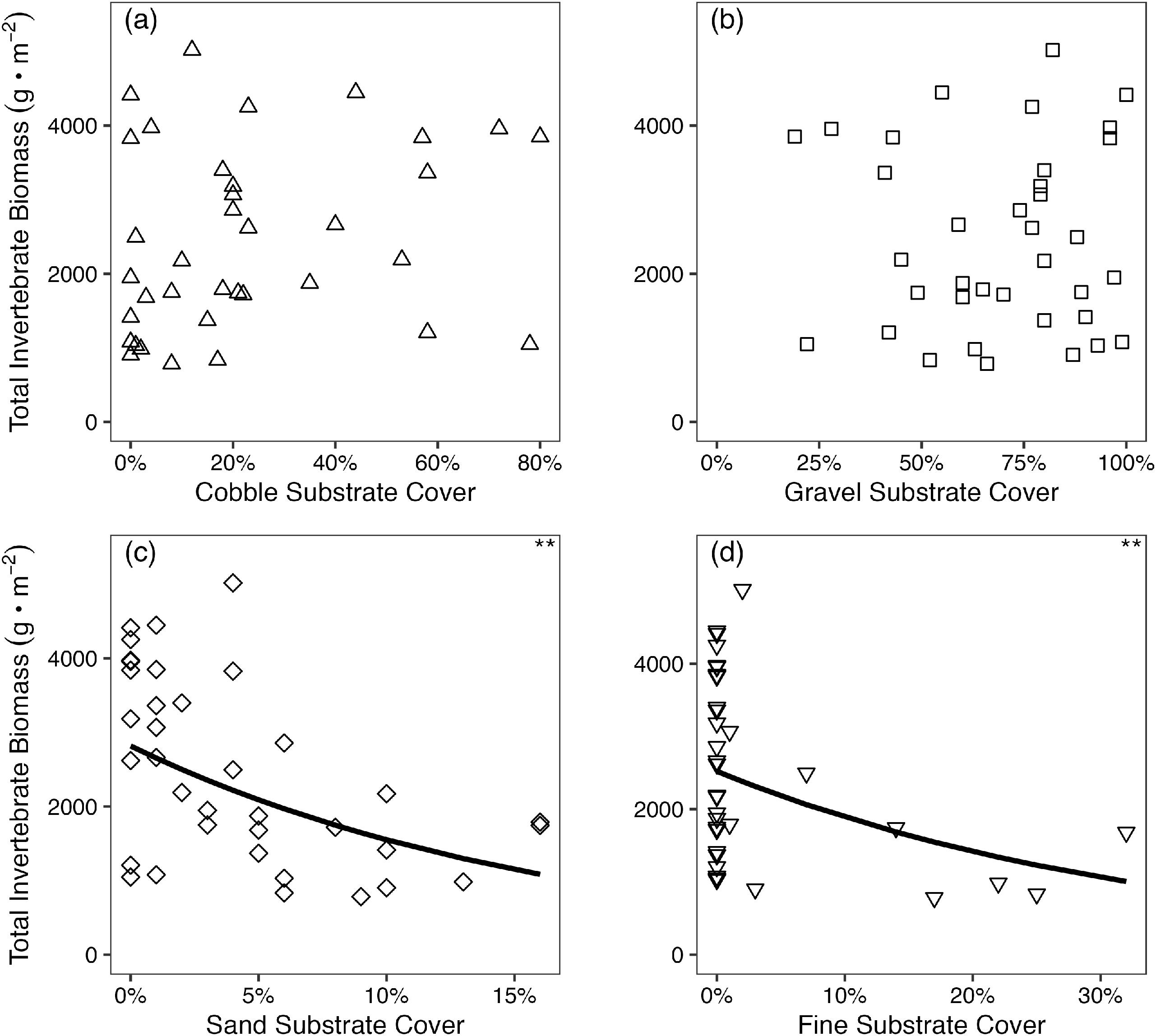
Benthic invertebrate data do not support the inference of enhanced dace abundance from increased benthic invertebrate prey biomass in cobble substrate, or a correlation between invertebrate prey biomass and interstitial space. This suggests that the primary mechanism whereby cobble affects Nooksack Dace is through direct use of interstitial habitat, rather than increased prey abundance. This is supported by significant correlations between the area and density of interstitial space and the maximum size and density of dace, respectively. However, this conclusion must be tempered by the large variation in benthic invertebrate biomass in all substrate treatments (Fig. S5), and the correspondingly low power to detect differences in benthic invertebrate biomass between gravel and cobble treatments, and controls. Many studies have demonstrated higher benthic invertebrate biomass on coarser substrate (e.g.,
Burdon et al. 2013;
Scholl et al. 2022), and there was a detectable negative correlation between benthic biomass and areal coverage of fines and sand in this study (
Fig. 6). Both Bertrand Creek and Pepin Creek are mildly eutrophic streams with high algal production, which tends to generate high benthic macroinvertebrate abundance (
Moore and Palmer 2005;
Bernot et al. 2006;
Herringshaw et al. 2011). Generally high benthic invertebrate biomass may mask any relationships between interstitial space and invertebrate biomass that might manifest in a more oligotrophic system.
Overall, this study confirmed that Nooksack Dace rely on unembedded interstitial spaces in coarse substrate, presumably for refuge from flow and predation, for spawning, and for foraging (
Pearson et al. 2007;
McEwan and Joy 2014;
Kawanishi et al. 2015;
Champion et al. 2018). The observation of reduced dace abundance when these interstitial spaces are lost is broadly consistent with the effects of sediment deposition seen elsewhere (
Wood and Armitage 1997;
Rambaud et al. 2009;
Descloux et al. 2013). Unexpectedly, the riffle restoration experiment also coincided with record fall floods that took place in November 2021 after the initial dace baseline sampling, but before the terminal sampling in August 2022. Bertrand Creek received peak flows approaching 50 m
3·s
−1 (unpublished data), raising concerns over potential Nooksack Dace mortality associated with bedload movement that could confound the experimental design. However, there was no reduction in dace density following the floods, despite evidence of significant bedload movement, indicating that Nooksack Dace are well-adapted to coping with high discharge events and bed instability. The increase in interstitial refuge area and density in control substrate treatments in the second year suggests that the flood exported fine sediment and reduced overall embeddedness. While this effect could confound the experimental treatment to some extent, the lack of a dace response in gravel and control treatments suggests that the overall reduction in fines was not consequential for the outcome of the study.
An increase in fish density following habitat restoration can be caused by one (or all) of three mechanisms. First, creation of higher quality habitat can lead to immigration of fish from adjacent lower quality habitat (e.g., dace may abandon riffles with highly embedded substrate and move to newly created riffles with abundant interstitial space;
Roni 2019). This aggregation response does not necessarily demonstrate an increase in total population size, despite the local increase in fish density in restored habitat, because it could simply represent a redistribution of fish. However, net immigration to the newly created habitat and higher fish densities relative to pre-restoration supports the inference of higher quality habitat at the restored sites, assuming that fish follow an ideal free distribution (i.e., that density reflects habitat quality;
Kennedy and Gray 1993). If the fish population in question is not recruitment limited (i.e., there are adequate juvenile fish to saturate adult habitat;
Rosenfeld and Hatfield 2006), then it is reasonable to assume that any lower quality habitat vacated by immigrants would be filled by recruits, resulting in a net increase in population size and carrying capacity. In contrast, if the population is severely recruitment limited, then increasing adult habitat may not result in a population increase, even if fish relocate to restored habitat.
Second, increased growth and survival in higher quality restored riffles could cause increased fish density (i.e., through lower mortality) and increase overall population size (
Roni 2019), even without immigration of fish to restored habitat. However, any population-level effect through this mechanism would likely be smaller than for a population with abundant recruitment to saturate both new and existing habitat. Finally, if reproduction takes place in the restored riffle habitat (as might be expected with Nooksack Dace), then enhanced recruitment would complement increased adult density or growth, and maximize the likelihood of a rapid population response to restoration. Young-of-the-year Nooksack Dace were present in all sampled riffles in Bertrand Creek, indicating that in situ recruitment may have contributed to increased densities in restored riffles (although young-of-the-year fish could also have originated from unrestored riffles). In contrast, while Pepin Creek riffles clearly showed a strong increase in dace density, no young-of-the-year recruits were observed in any of the restored (or control) riffles over two years of sampling in Pepin Creek (
Gray 2023). This indicates that the higher post-restoration density in Pepin Creek was entirely an aggregation/immigration response, and is unlikely to reflect a net increase in population size given the extreme recruitment limitation that we observed. This serves as a cautionary note that increased density following habitat restoration may not always be an accurate diagnostic of successful population recovery, and highlights the importance of considering the mechanism(s) underlying locally increased density. Low and intermittent recruitment is clearly limiting the Nooksack Dace population in Pepin Creek, and simple riffle restoration failed to alleviate this, at least for one year of post-restoration monitoring. Loss of juvenile rearing habitat due to encroaching reed canarygrass is implicated in juvenile recruitment failure in Pepin Creek (
Gray and Rosenfeld 2024), where riffle restoration will remain ineffective in population recovery until the juvenile recruitment bottleneck is addressed.
This study integrated a structured BACI habitat restoration experiment with subsidiary analyses to identify the mechanisms driving abundance of Endangered Nooksack Dace, and to evaluate the practical effectiveness of riffle restoration. While replicates were limited by logistic challenges (i.e., resources, time, and space) as is often the case for field studies, the strong and consistent treatment effects suggest that the results are robust and biologically relevant. The distribution of Nooksack Dace in Canada is limited to only four streams, with the majority of fish residing in the two study streams, so that the observed dynamics are directly relevant to over half of the total Nooksack Dace population in Canada. The reaches where experiments were conducted were also highly representative of general habitat conditions in each study stream. And by encompassing the full range of habitat quality from very high dace densities to strongly recruitment limited, the experimental design ensured a broad scope of inference and generality across a wide range of habitat contexts.
Restoring degraded habitat represents one potential technique to reverse the effects of anthropogenic disturbance by recovering habitat that has been degraded, damaged or destroyed (
Rey Benayas et al. 2009). Significant increases in Nooksack Dace density following riffle restoration indicates that riffle restoration does indeed represent a useful tool in the recovery of an endangered riffle specialist, but the overall role of this intervention in species recovery needs to be kept in perspective, for two reasons. First, riffle restoration will only be effective at increasing population size in streams where (i) recruitment is sufficient to saturate restored habitat and (ii) in streams where any net gain in habitat through restoration is not negated by an equivalent habitat loss elsewhere (
Maron et al. 2018). The management paradigm of no net loss of habitat (
Harper and Quigley 2005) is based on the reasonable inference that populations will decline if habitat loss exceeds habitat gain. The ultimate effectiveness of local habitat restoration in recovering populations is predicated on the assumption that habitat loss elsewhere is negligible. Habitat restoration to compensate for ongoing habitat destruction elsewhere is generally not a sustainable or effective routine management strategy (
Harper and Quigley 2005;
Moreno-Mateos et al. 2015) because the rate of habitat loss generally exceeds capacity to restore degraded habitat (
Maron et al. 2012,
2018). Second, instream habitat restoration, even when locally effective, takes place in a watershed context (
Beechie et al. 2010) where cumulative basin-scale impacts can reverse local gains. Of particular concern in Nooksack Dace watersheds are increases in water temperature and hypoxia associated with eutrophication from cumulative landscape-scale nutrient enrichment and climate change, which can easily render restored reaches uninhabitable for the target species (
Rosenfeld et al. 2021). To be effective, instream habitat restoration must be combined with watershed-scale management to ensure that restored habitat remains functional, and is part of a larger integrated recovery strategy. In the absence of wider habitat protections, restored habitat may eventually be degraded or destroyed, as has occurred with riffle habitat throughout the LFV (
Boyle et al. 1997;
Pearson et al. 2007).