Introduction
Biological indicators (“bioindicators”) include species, communities and/or organismal processes used by monitoring and management programs to detect change in population and ecosystem attributes, including ecological functions. These may range across different scales, from habitat forming species to entire ecosystems (
Carignan and Villard 2002). Effective bioindicators are generally (1) sensitive to changes in the environment, while remaining stable in response to natural spatial and temporal variability; (2) occupy a temporal and spatial distribution for widespread implementation and comparison; and (3) are feasible and cost-effective to consistently measure (
Carignan and Villard 2002;
Manickavasagam et al. 2019). Identifying and quantifying the magnitude of directional change and variability in biological indicators in response to anthropogenic stressors or management actions requires assessing the relationships between bioindicators and environmental conditions. Given interannual variability in environmental conditions, multiple years of concurrent environmental data and bioindicator monitoring are often needed to establish these relationships in situ (
Morris et al. 2008). Furthermore, understanding the effects of environmental conditions on bioindicators may require advanced statistical modeling of the relationships (
Compagnoni et al. 2021). This necessary step is difficult to establish in challenging coastal and marine ecosystems, where in-situ data are logistically challenging to collect.
Given the ecological importance and recent local declines in kelp forests around the globe, there is growing interest in using kelp, and metrics of kelp, as bioindicators of environmental change in marine ecosystems (e.g.,
Marine Planning Partnership Initiative 2015—Central Coast Marine Plan). Kelp habitats have been shown to be increasingly sensitive to climatic conditions such as temperature, including temperature anomalies and marine heatwaves, long-duration climate-driven fluctuations (e.g., ENSO), and gradual climate warming (
Reed et al. 2016;
Cavanaugh et al. 2019;
Wernberg et al. 2019;
Starko et al. 2022). These documented changes in response to climate also impact the ecological functions of kelp forests, including their global role in primary productivity (
Mann 1973). Kelp biomass and productivity support diverse assemblages of invertebrates, fishes, mammals, and birds, including many important fisheries and at-risk species (e.g.,
Mann 1973;
Steneck et al. 2002) and serve an important role in nutrient cycling, including nitrogen and carbon uptake via photosynthesis (
Teagle et al. 2017;
Eger et al. 2023). In Canada, for example, bioindicators are being assessed for monitoring and management of marine ecosystems, led by kelp forest programs among Indigenous stewardship, First Nations and the Province of B.C. (
Marine Plan Partnership or MaPP for the North Pacific Coast—Central Coast Annual Report 2020), as well as ecologically significant species (ESS) within ecologically and biologically significant areas (EBSAs) for Fisheries and Oceans (
Rubidge et al. 2020).
The use of kelps as bioindicators has led to additional questions about which demographic or functional properties (e.g., kelp growth, biomass, productivity) are most sensitive to environmental change, and therefore serve as the “best” bioindicator. Kelps exhibit rapid rates of growth and recruitment, which are sensitive to seasonal and interannual changes in climate, and result in high biomass turnover and rapid shifts in productivity (e.g.,
Krumhansl et al. 2016;
Wernberg et al. 2019). Consequently, changes in these rates can be used as indicators of interannual and seasonal changes in kelp productivity and health in response to climate-influenced abiotic conditions (
Compagnoni et al. 2021). Kelp population-level change in response to the marine environment usually uses information on extent (often for canopy-forming species) and abundance (e.g., densities or % cover). However, demographic parameters or vital rates (e.g., recruitment, mortality and survival) may provide ideal indicators of overall population success. Kelp growth, which is particularly efficient and cost-effective to measure and monitor in situ over time, may therefore serve as an important bioindicator to assess population-level shifts in response to changes in environmental conditions.
In the northeast Pacific, recent collapses in annual populations of
N. luetkeana as well as other species of kelps are attributed not only to shifts in herbivory but also to rapid changes in the abiotic conditions that support them, partly as a result of declines in growth (e.g.,
Berry et al. 2021;
McPherson et al. 2021;
García-Reyes et al. 2022;
Starko et al. 2022). The combined impacts of multiple stressors on kelp, however, can be context specific to kelp life history strategies and the local coastal environment. Kelp declines have been attributed to changes in both abiotic and biotic conditions, including rising sea surface temperatures and anomalies (
Wernberg et al. 2013), changes in nutrients and light regime associated with turbidity (
Coleman et al. 2008), increasing wave action and storms (
Smale and Vance 2016), and loss of predators and overgrazing by herbivores (e.g.,
Burt et al. 2018). In many, if not most coastal settings, multiple stressors co-occur and influence kelp populations (e.g.,
Burt et al. 2018;
Rogers-Bennett and Catton 2019).
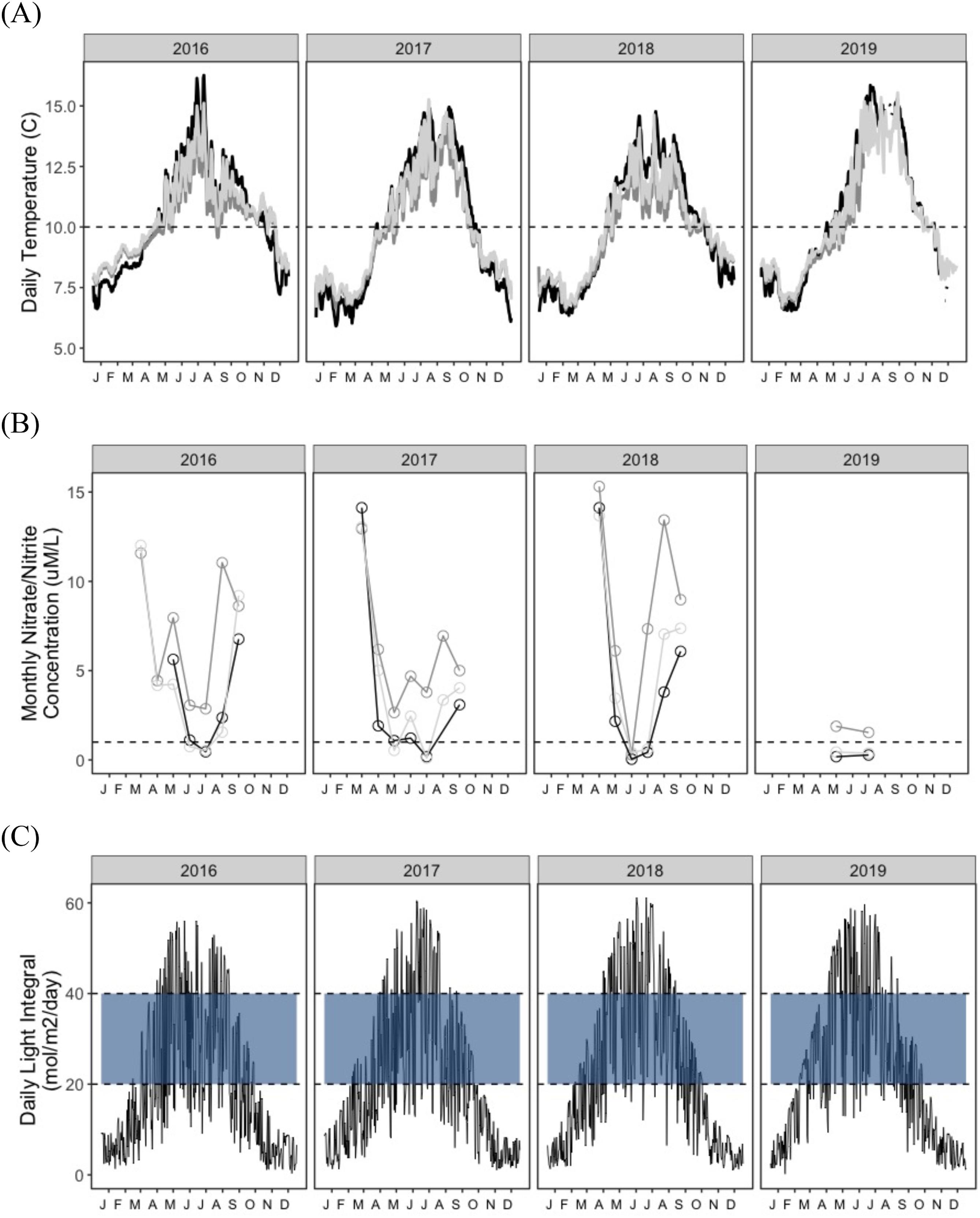
N. luetkeana experiences a wide range of environmental conditions across its broad geographic distribution from central California to subarctic waters of southeast Alaska, which spans a latitudinal temperature gradient of 4 °C to 15 °C (
Lüning and Freshwater 1988). Driven by weather patterns, currents and local geography, microclimates also result in substantial variability in temperature, nutrients, and light of
N. luetkeana (
Foreman 1984). Field observation has documented declines in abundance (
Berry et al. 2021) and in photosynthetic performance (
Wheeler et al. 1984) in association with changes in abiotic factors such as temperature and dissolved inorganic nitrogen (nitrate). Laboratory studies also suggest that
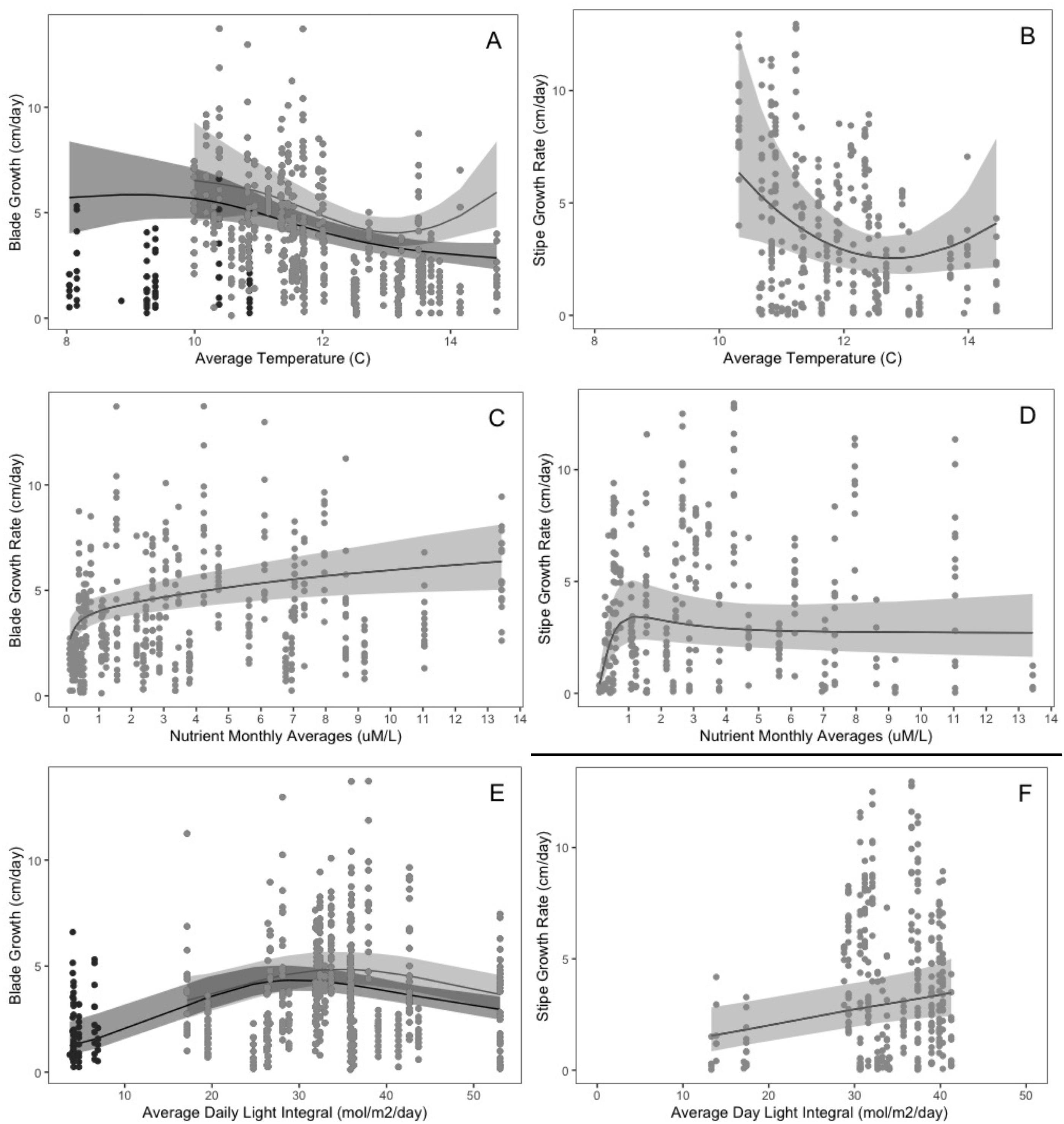
N. luetkeana growth is sensitive to small changes in temperature (
Supratya et al. 2020;
Fales et al. 2023), nutrients (
Ahn et al. 1998), and light (
Wheeler et al. 1984). These variable local conditions likely influence
N. luetkeana growth dynamics across seasons and years (
Brown et al. 1997), as well as the sensitivity of its growth metrics to changing conditions (
Foreman 1984), and its ability to adapt to future changes associated with climate stress. Despite the importance of
N. luetkeana as a dominant canopy-forming kelp, the effects of specific abiotic conditions on its growth and productivity have not been widely characterized, except for the few focal studies above.
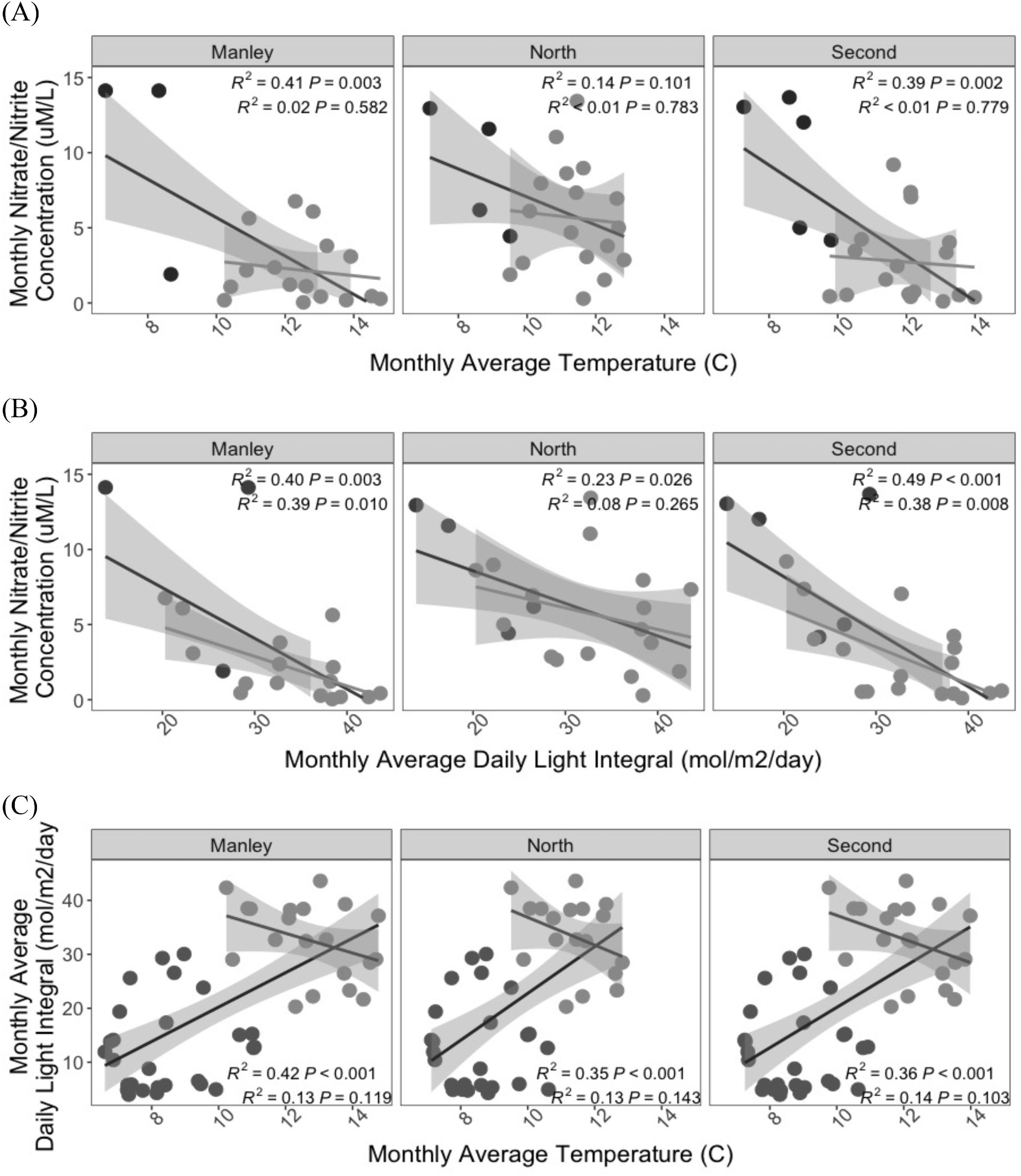
Patterns of persistence in
N. luetkeana and other kelps in BC may also differ from those of other regions due to differences in climatic conditions during their primary growing season. Temperature and nutrients in California are correlated through the primary growing season of the dominant canopy-forming perennial kelp,
Macrocystis pyrifera (e.g.,
Zimmerman and Kremer 1984), and both sea surface temperature and nutrient availability are related to the annual recovery of this kelp species in the early spring, with weaker negative relationships spanning the full year (
Cavanaugh et al. 2011). As observed through a 25 year times series in California, interannual relationships between SST and other climate indices (e.g., NPGO) and kelp-canopy biomass were lagged (
Cavanaugh et al. 2011). In contrast, in British Columbia, the relationships between temperature and nutrients are not well established, in part because of different upwelling dynamics along a high rugosity coastline. Upwelled waters interact with land-based inputs from coastal watersheds (
St. Pierre et al. 2021), resulting in local variation in temperature-nutrient relationships (
Ebbesmeyer et al. 1988). As a result, the interactive effects of temperature and nutrients on the dynamics of kelp indicator metrics may depend on the local seasonal and interannual variability of the region. However, data to examine these relationships is limited, as there are few multi-year time series of in-situ kelp demographic data and environmental data for the Pacific Coast of Canada (except see
Watson and Estes 2011). Understanding these critical thresholds for growth indicators, and the inflection points where the greatest magnitude of change in kelp response is observed, is critical in natural settings (
Jackson 1977;
Foreman 1984;
Dayton et al. 1999) especially for canopy-forming species that are difficult to maintain and manipulate in laboratory settings.
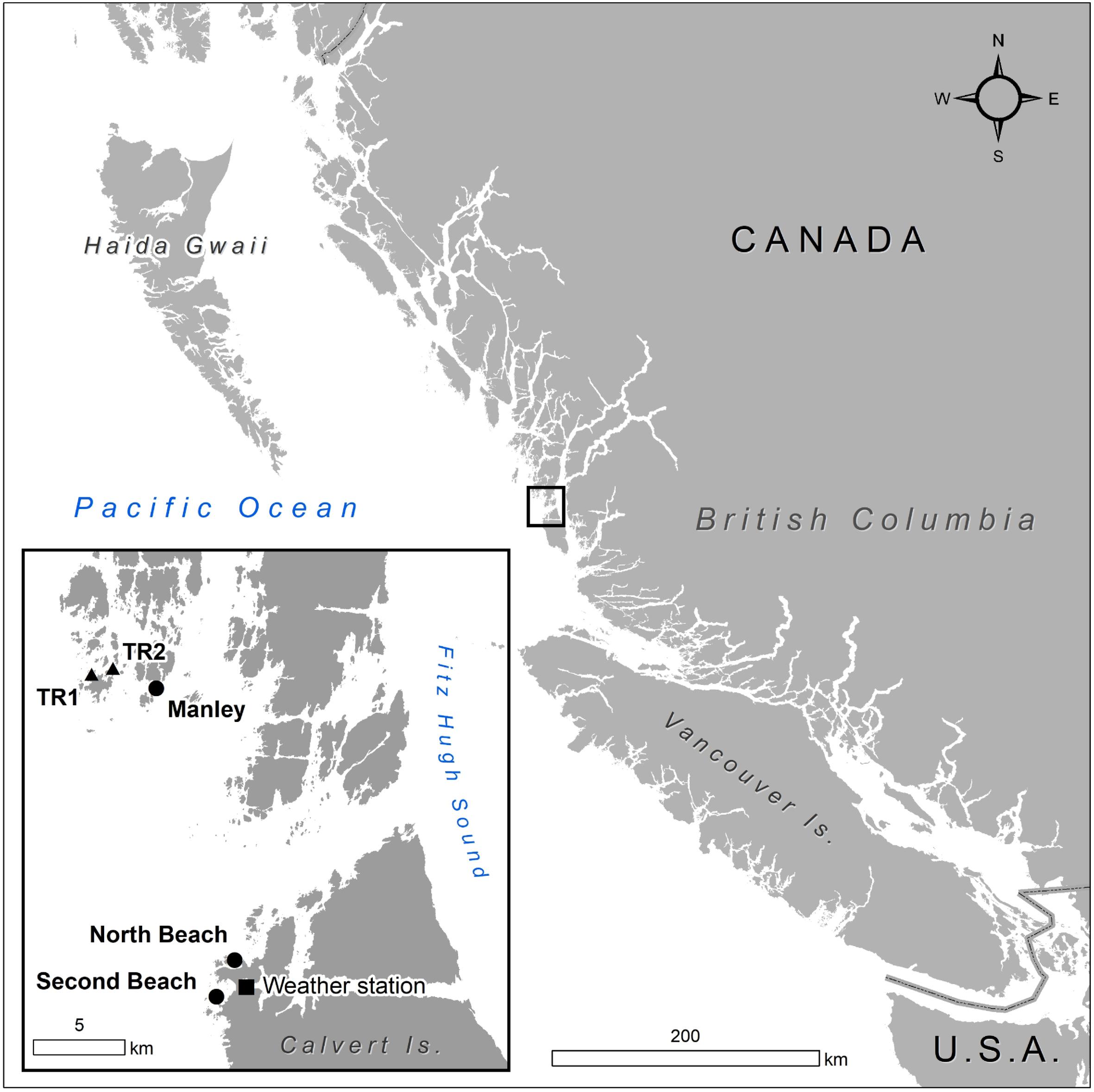
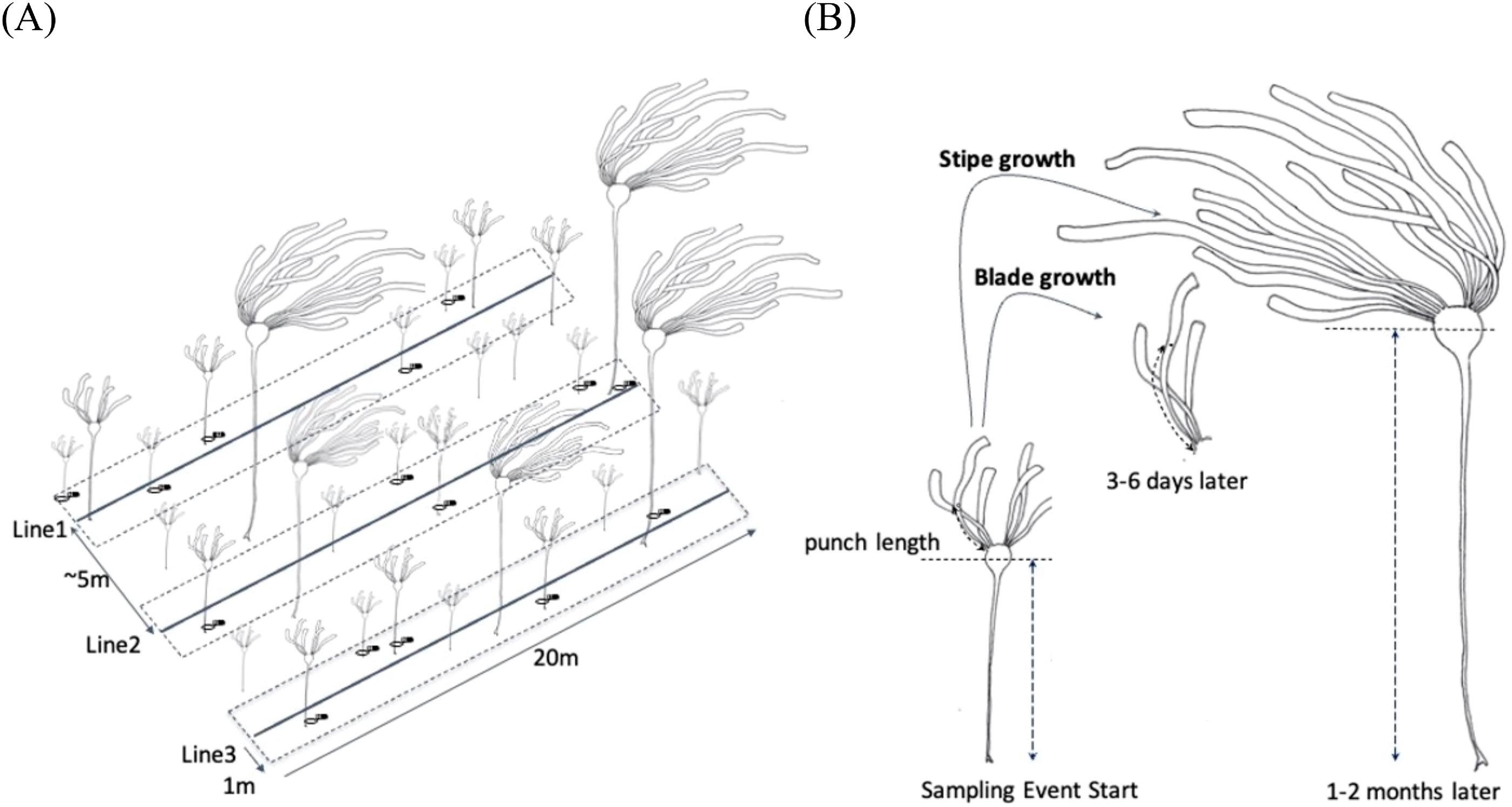
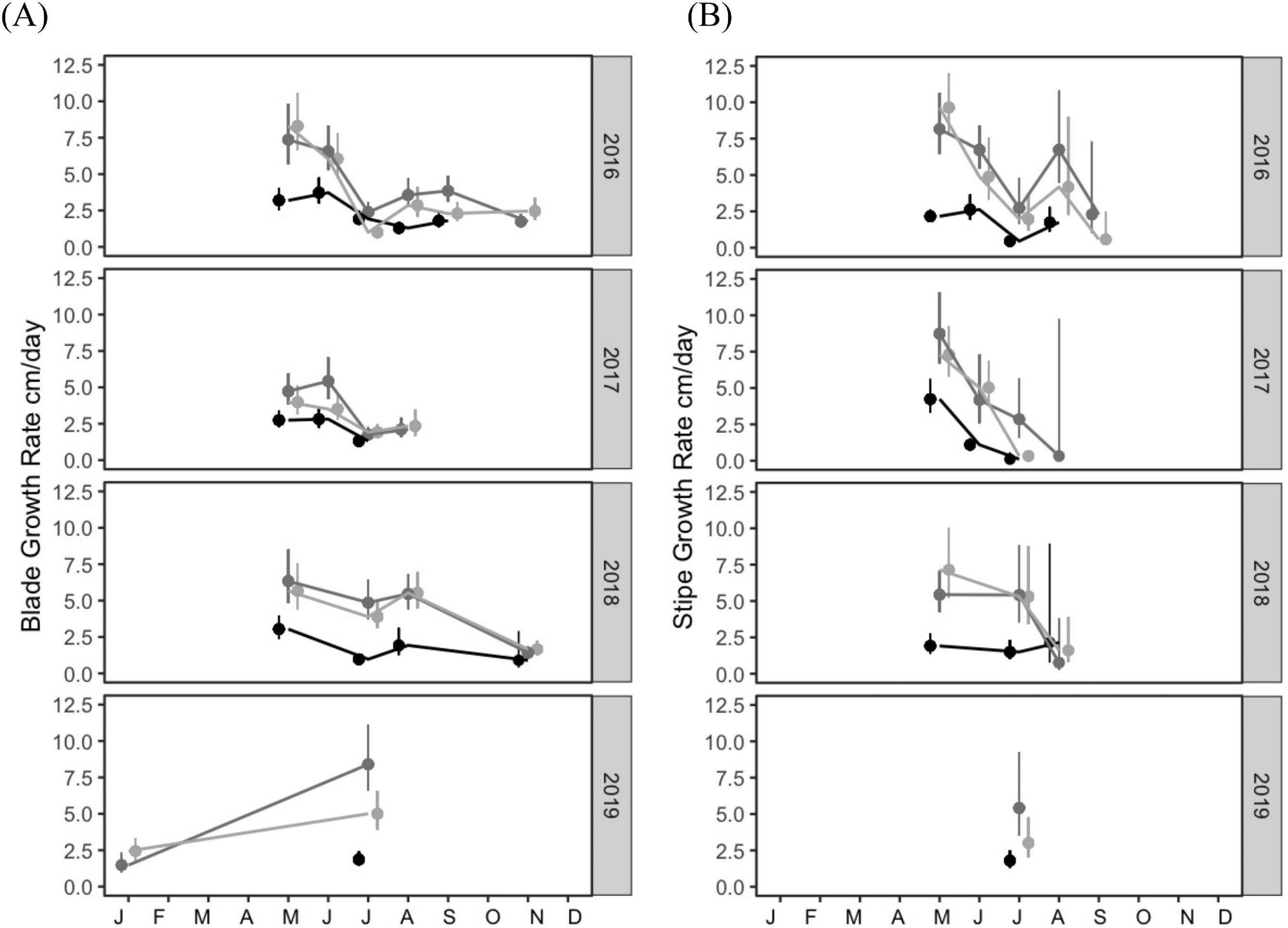
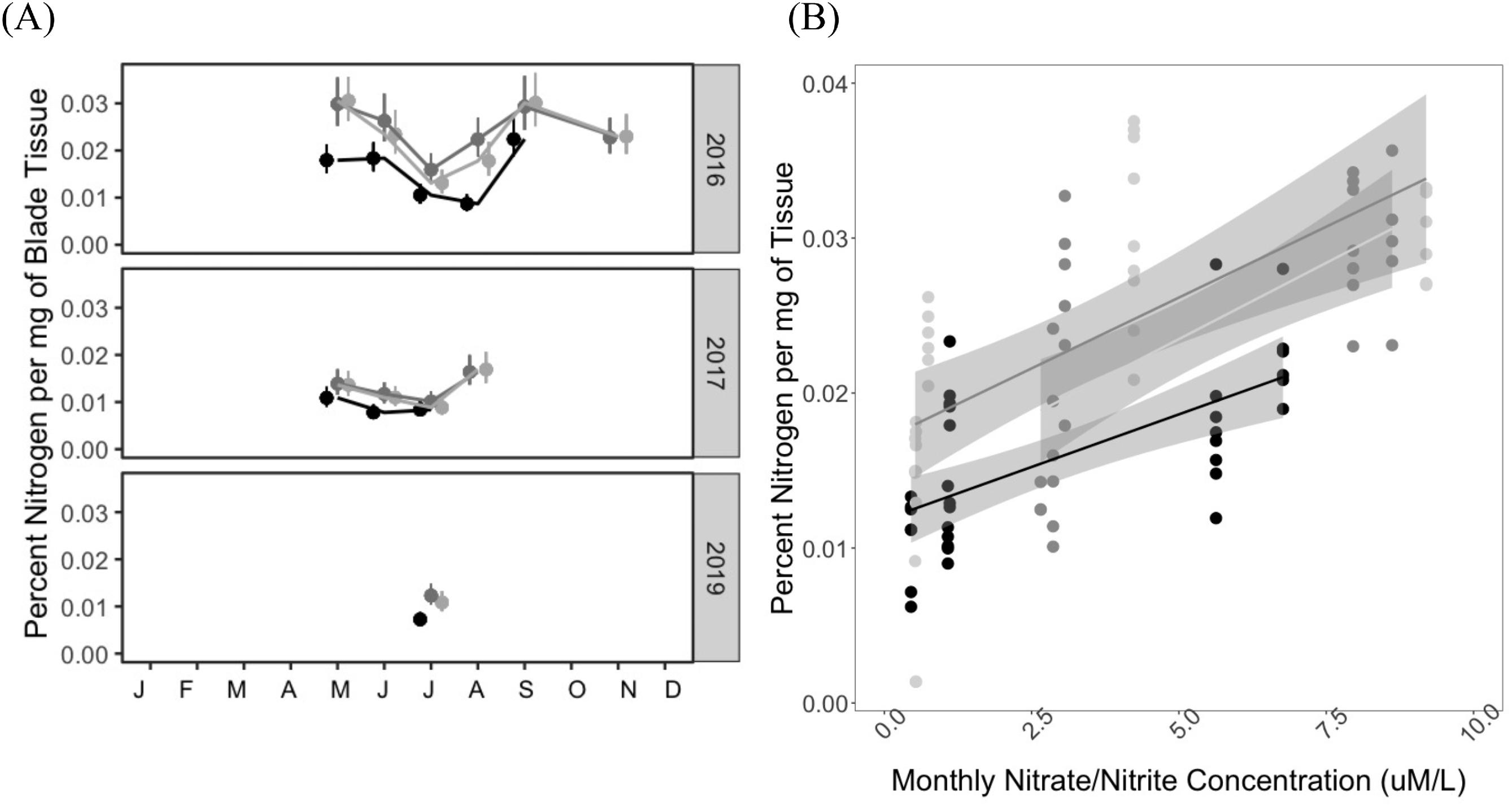
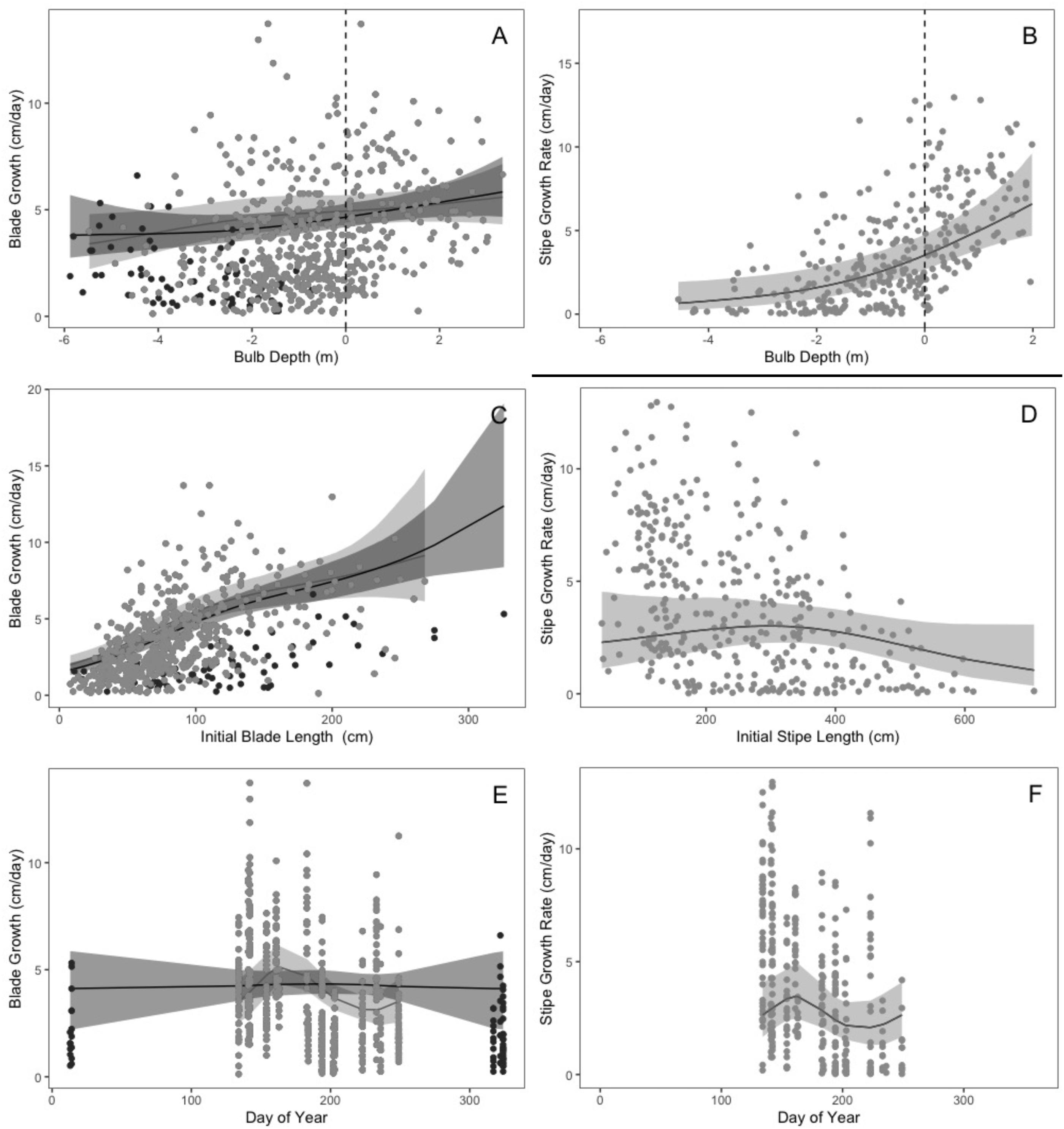
In this study, we aimed to establish (1) the baseline range and spatiotemporal variability for indicators of kelp productivity, and (2) the key environmental factors that covary with these indicators. To assess indicators of
N. luetkeana productivity we measured two key growth metrics (blade and stipe growth) in situ over four years (May 2016 to July 2019) at three sites on the Central Coast of BC. We then established specific relationships between growth rates and important abiotic/biotic factors (Supplementary Materials, Table S1). To assess how climatic factors affect individual growth dynamic across seasons, years, and sites, we measured how temperature, nutrients, and light availability changed and co-varied over seasons, years, and sites and especially, across the primary growing season. We modeled the relationship between growth and each environmental factor while accounting for inherent phenology through proxy processes such as size and seasonal trends, and for each trajectory determined the inflection points of high sensitivity (where % change in growth per change in predictor variable is maximized), as well as their thresholds to growth (upper and lower bounds). Through this indicator assessment, our goal is to establish this parameter’s effectiveness in signaling climate stress to kelp, and to inform the feasibility of implementing this monitoring metric in the context of management, protection/planning, and restoration of this important canopy-forming annual macrophyte species (
McPherson et al. 2021).