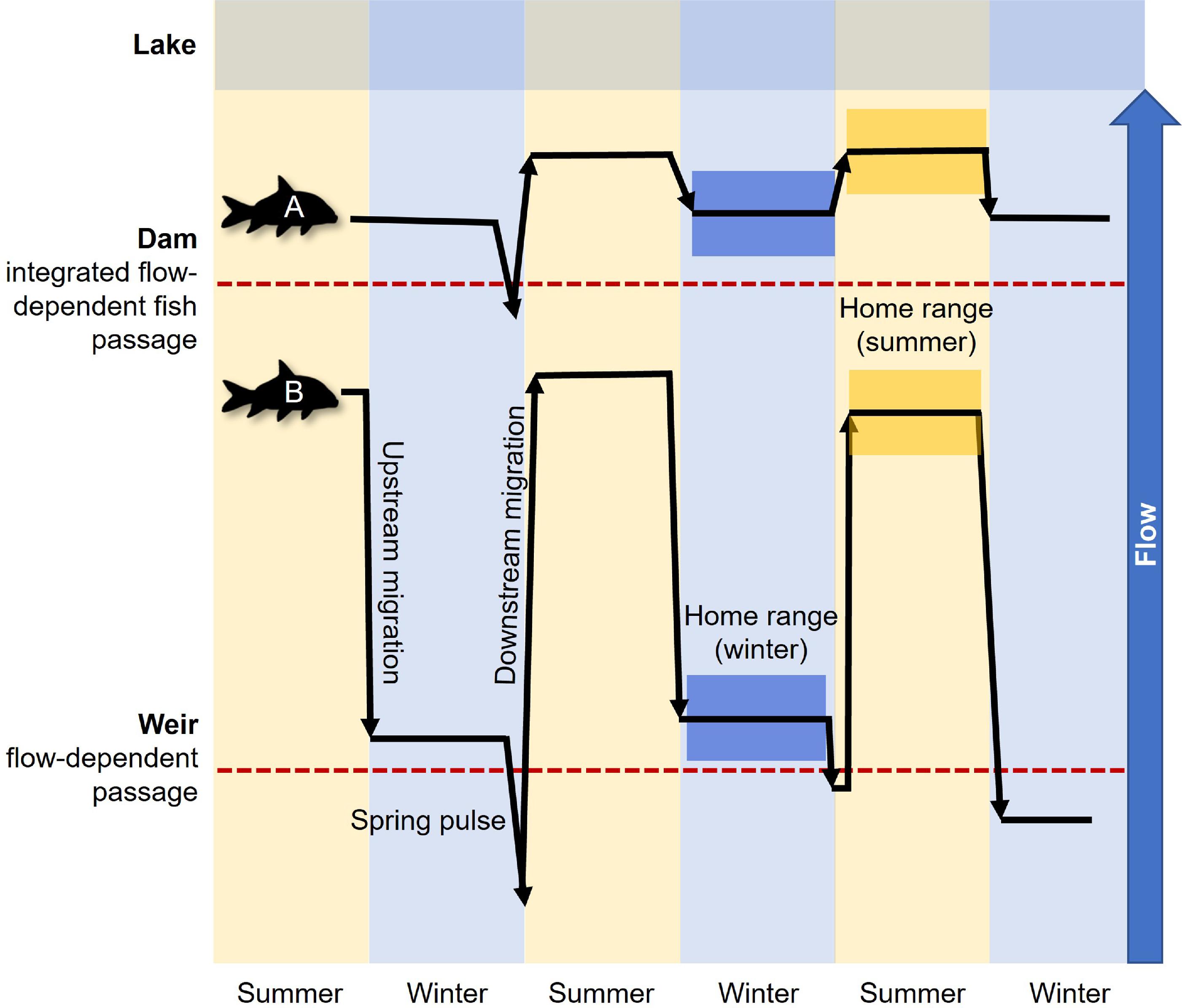
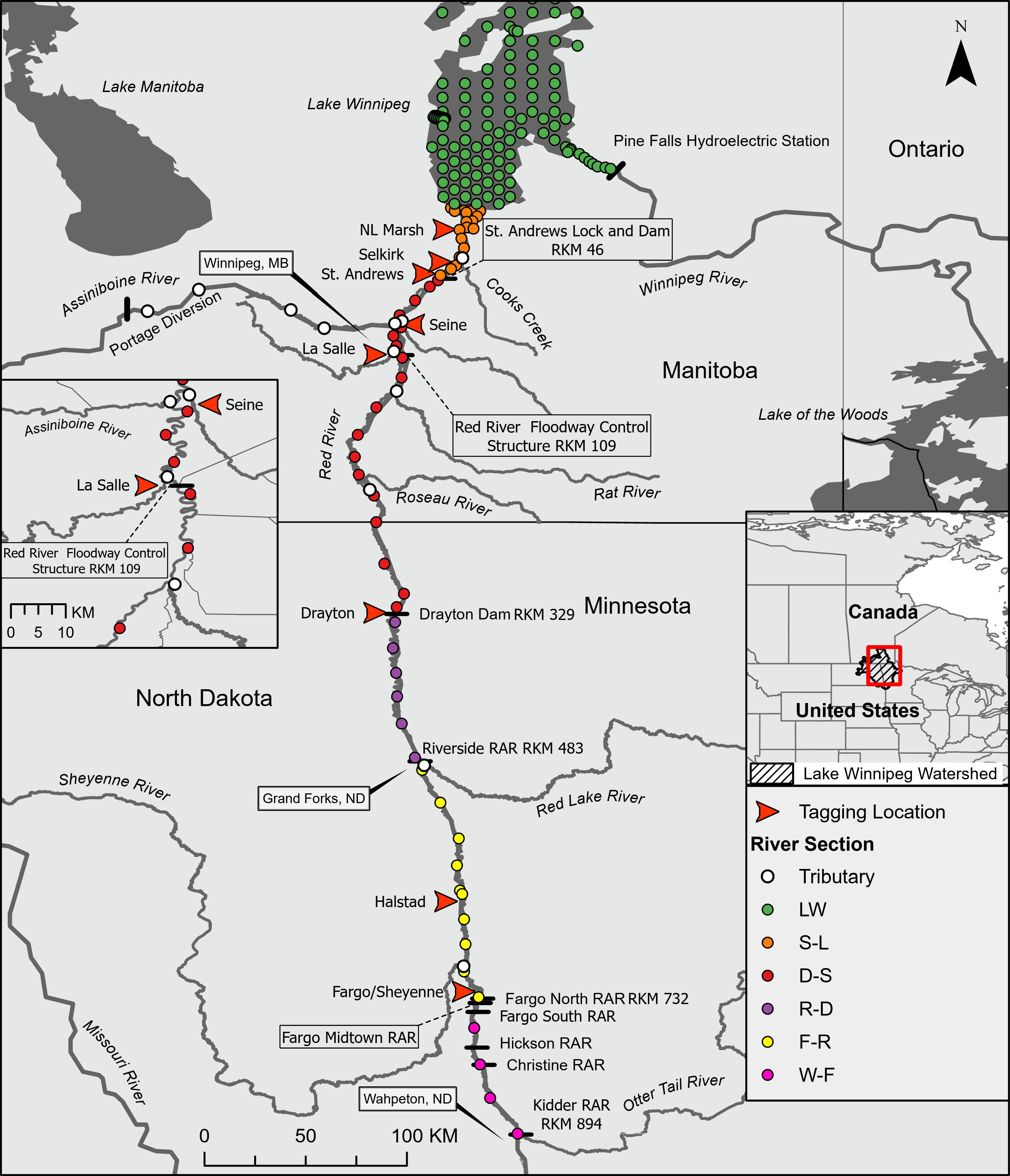
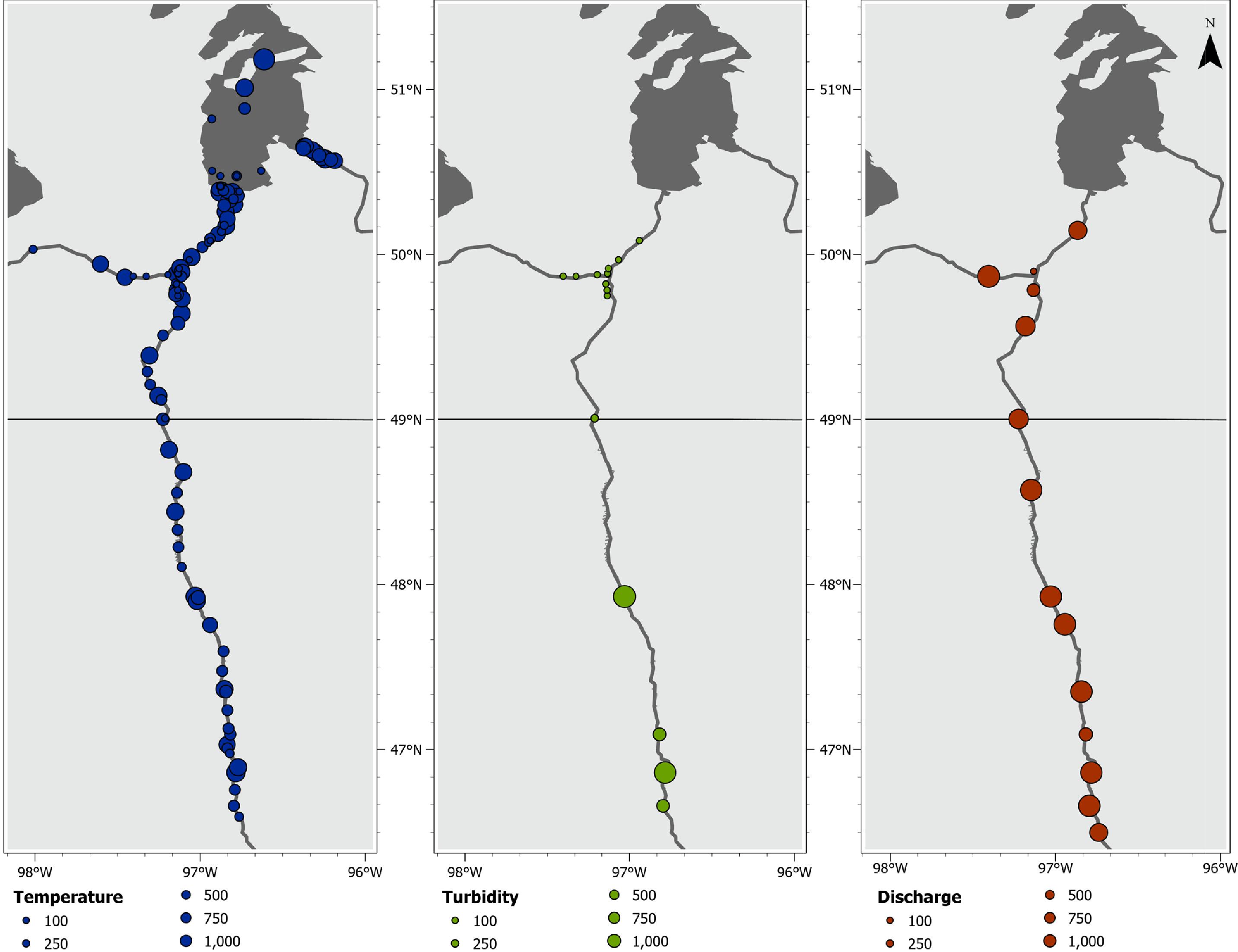
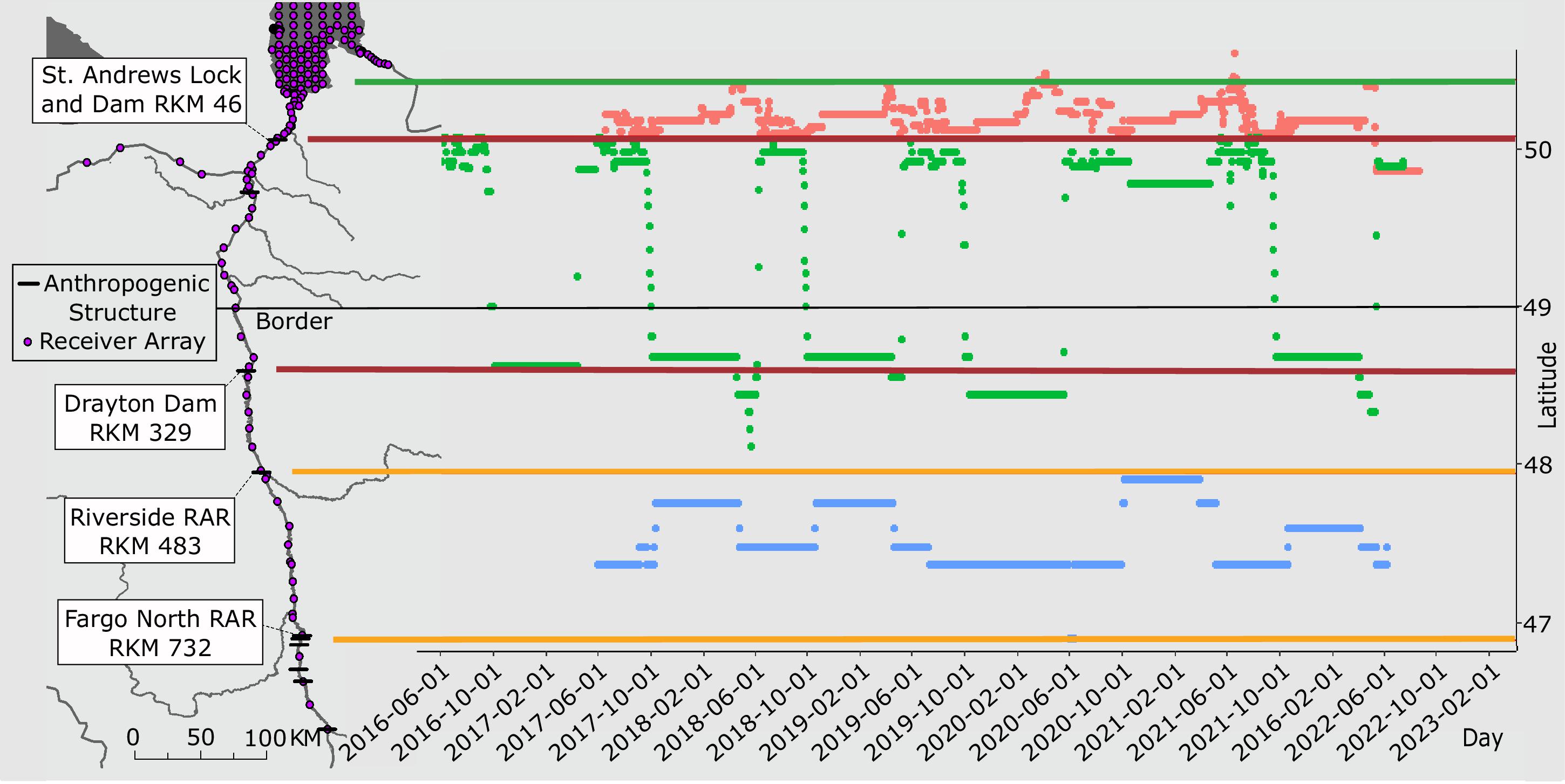
Introduction
Efforts to conserve migratory animals are routinely challenged by increasing anthropogenic disturbance and limited baseline data on habitat patch use (
Bauer et al. 2016;
Cohen et al. 2018;
Marra et al. 2019). Migration is an essential component of life history strategies in heterogenous environments, yet access to high-quality habitat may be limited if disturbance impacts structural connectivity. As disturbance often precedes initial data collection, conservation planning must rely on the status quo or establish quasi-baselines (
Franklin et al. 2024). Information on habitat patch use is especially salient to conservation efforts directed towards migrants that are impacted by climate change and at risk because of severed connectivity (
Nuñez et al. 2013;
Keeley et al. 2021).
In aquatic ecosystems, barriers such as dams and weirs disrupt both longitudinal and lateral connectivity within and among networks of flowing waters and adjacent habitats (
Grill et al. 2019). This impedes the flow of nutrients and aquatic organisms to fundamentally alter the environmental conditions to which species and populations have adapted. These barriers confine aquatic organisms to fragments of a river network, limiting the accessibility of geographical space and therefore hampering the ability to move between seasonally changing habitats with different ecological niche conditions (
Rahel and McLaughlin 2018;
Franklin et al. 2024). Ultimately, the interplay between barrier positioning and habitat heterogeneity influences accessibility to habitat with seasonally-specific suitability.
Bigmouth Buffalo (
I. cyprinellus) is a potamodromous North American freshwater fish and the largest member of the family Catostomidae. It is the only catostomid to use a filter-feeding strategy and strain plankton from the water (
Stewart and Watkinson 2004). The species currently holds the record for oldest teleost, with the most recent maximum age estimate of 127 years (
Lackmann et al. 2023). As with other periodic life history strategists (
Winemiller and Rose 1992), Bigmouth Buffalo have delayed maturity, broadcast spawn with no parental care, reproduce infrequently, and exhibit high fecundity (
Lackmann et al. 2019). At the northern extent of its range in south-central Canada, reproduction occurs from late-May to early-June in shallow marshe
s, flooded riverbanks, or lake shores (
Stewart and Watkinson 2004). Precipitous declines in Canadian populations during the mid-late 20th century have been attributed to commercial fishing, habitat fragmentation, and potential competition with naturalized Common Carp (
Cyprinus carpio;
Goodchild 1989). In response, the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assessed the Saskatchewan-Nelson River populations as “species of concern” (
COSEWIC 2009). Shortly thereafter, Bigmouth Buffalo was listed as Special Concern under the federal Species at Risk Act in 2011 (
Fisheries and Oceans Canada 2020).
Bigmouth Buffalo occupy several watersheds across North America where dams and barriers are ubiquitous (
Barbarossa et al. 2020). In one of the few riverine systems where Bigmouth Buffalo movement has been assessed, the Red River of the Hudson Bay Basin, home ranges can exceed hundreds of kilometers in undisrupted stretches of river (
Enders et al. 2019). Where rivers span degrees of latitude and resource availability changes seasonally, habitat fragmentation is likely to disrupt migratory behaviours (
Fretwell and Lucas 1970;
Fretwell 1972). Examining how and when Bigmouth Buffalo occupy habitats in fragmented systems is a logical step toward uncovering the factors impacting an already imperiled species.
To better understand the effects of river fragmentation on Bigmouth Buffalo migration and seasonal home ranges, we examined 6.5 years of passive telemetry data collected in the Red River watershed. Specifically, we investigated the conditions under which Bigmouth Buffalo moved between summer and winter home ranges, and the overlap between these ranges, across river sections characterized by varying lengths. Due to the potential for seasonally changing habitat suitability and high habitat heterogeneity in the river network (
Enders et al. 2019), Bigmouth Buffalo should exhibit distinct summer and winter home ranges (i.e., minimal overlap). Because Bigmouth Buffalo have large home ranges but limited dam passage in this river system (
Enders et al. 2019), we hypothesized that the degree of seasonal home range overlap is a function of unrestricted geographic space as delineated by river section. In this context, the probability of home range overlap will be minimized where Bigmouth Buffalo are most at liberty to find the seasonally available resources and conditions that maximize fitness among the heterogenous riverscape. This research is intended to enhance the long-term survival of Bigmouth Buffalo by uncovering consequences of habitat fragmentation, thereby informing effective management strategies for the species.
Discussion
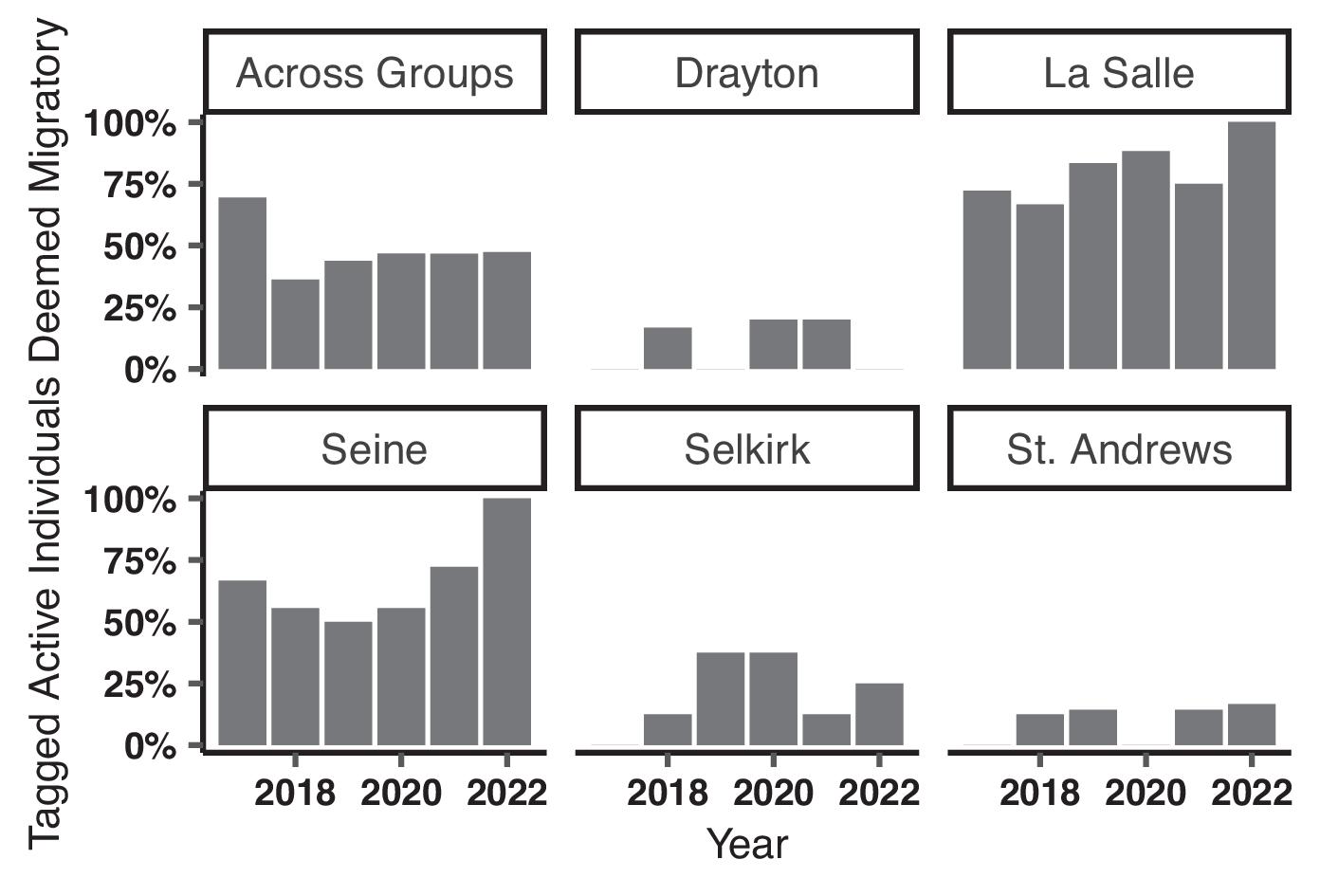
Long-term monitoring of individual Bigmouth Buffalo movements in the Red River revealed notable differences in migratory behaviour and the use of distinct seasonal home ranges across river sections in a fragmented river network. The comparison of seasonal home range overlap and size between river sections of different lengths indicated an upper and lower bound to summer home range sizes for Bigmouth Buffalo. These findings add to the limited body of information available to resource managers tasked with conserving or restoring Bigmouth Buffalo and its habitat.
Many spring migrations, likely coinciding with reproduction (
COSEWIC 2009), started with a rapid increase in water temperatures and higher river discharges (
Fig. 5). The downstream destination was the most repeatable behaviour estimated (
Table 1), and observations of spawning activity and sexually mature fish in the La Salle River support the assertion that Bigmouth Buffalo use this tributary for spawning (Watkinson DA, personal observation). There was high individual variation in the start and end dates of spring migration (
Table 1), with many fish departing several weeks later in the season. By this time, summer water temperatures had nearly peaked and spring flows were considerably decreased (
Fig. 5). Low variability in the timing of spring spawning migration is generally considered necessary to derive fitness benefits from enhanced breeding success (
Hulthén et al. 2022 and refences therein); however, a number of species, particularly those considered long-lived or burdened by high reproductive costs (e.g., long migrations), will skip spawning as a survival tactic to offset mortality (
Jørgensen et al. 2006;
Lowerre-Barbieri et al. 2011;
Rideout and Tomkiewicz 2011;
Gallagher et al. 2018). Among the longest-lived teleosts, Bigmouth Buffalo are known to skip spawning (
Lackmann et al. 2021;
Lackmann et al. 2023). If this occurs, migration can still provide lifetime fitness benefits if the broader destination serves as the summer feeding grounds. Moreover, individuals that failed to migrate at all (
Fig. 4) show that partial migration also occurs in the system (
Chapman et al. 2012). Documenting this phenomenon here is important, as partial migration can stabilize and bolster population resilience in heterogenous environments (
Kerr et al. 2010;
Chizinski et al. 2016;
van Leeuwen et al. 2016).
For spawning Bigmouth Buffalo, flood duration and timing may be among the primary drivers of the observed migration to downstream reaches where flooded riparian vegetation provides ideal habitat in the Red River. These spring conditions are both reflected by the environmental data (
Fig. 5) and relatively lower detection frequency observed during downstream migration (
Fig. 3). On average, the lower slope and higher discharge of the Red River near Winnipeg results in lower water level fluctuations relative to upstream reaches (
ECCC 2023). The peak of the spring flood begins in the US portion of the watershed and moves downstream, thus peaking in Canada later in the year. Flood duration is longer in Canada than in upstream reaches, despite less change in water surface elevation (
ECCC 2023). Water surface elevation of the Red River upstream of the La Salle and Seine rivers throughout the City of Winnipeg is influenced by SALD, which annually floods portions of the ephemeral river channels. Downstream of SALD, water surface elevation of the Red River may be further influenced by the seiche effect of Lake Winnipeg, resulting in capricious floodplain inundation
(ECCC 2023). This could result in Bigmouth Buffalo releasing eggs onto habitat that in hours or days becomes dry with changing water surface elevation. Common Carp have been observed spawning in flooded cattail (
Typha glauca) in Netley-Libau Marsh, where water depth was ∼70 cm but completely dry the following day. Larval stranding has been documented for Bigmouth Buffalo in Pound Lake, Saskatchewan (
Lackmann et al. 2023). In the Red River, Bigmouth Buffalo spawning downstream of SALD may still have reduced success in years when seiche effects on water surface elevation are high. Such changes to the duration and timing of flooding, in addition to surface temperatures, likely further explain some of the observed variability in migratory behaviour. Additional data are needed to understand flood impacts on behaviour and spawning success.
While reproduction is the most parsimonious explanation for spring migration in mature individuals, and appears to be driven by environmental cues, the fall upstream migration to overwintering habitat is more puzzling. Individuals typically departed from similar latitudes and concluded unidirectional movements around the same time each year (Julian day of upstream destination;
Table 1). Unlike the spring migrations, fall movements did not appear to be initiated by a clear environmental cue, besides decreasing temperatures (
Fig. 5) that were significantly lower at time of arrival compared to the onset of migration. The energetic benefits provided by overwintering habitat must outweigh the costs of moving. In the absence of a clear environmental cue or reproductive requirement, we hypothesize that the fall upstream migration is an adaptive strategy to align with a downstream progression, or “green wave,” of resources during the ensuing spring (
van der Graaf et al. 2006;
Bischof et al. 2012). Although suggested for migrating Arctic Char (
Salvelinus alpinus) that “jump” ahead of system productivity (
Hammer et al. 2022), this hypothesis is rarely applied to aquatic systems. Given the synchronization between plankton blooms and ice-off (
Hrycik et al. 2012), the earlier onset of ice-off upstream likely triggers a series of cascading downstream plankton blooms facilitated by increasing light availability and nutrients delivered by snowmelt (
Corriveau et al. 2011). During the fall, Bigmouth Buffalo seek the furthest upstream sites possible, partially blocked only by Drayton Dam, to capitalize on the most profitable spring plankton bloom. Annual discharge offers some support for this hypothesis, as Bigmouth Buffalo ended fall migrations downstream of the Drayton Dam every year except for 2019 and 2022, which coincided with unusually high fall flow events that made the dam passable upstream during the migration window (
Enders et al. 2019). Improved passage potential over Drayton Dam resulted in longer and more variable fall migration distances in 2019 than the other years. For example, in 2019 the mean upstream migration distance was 325 rkm (SD = 157) compared to the next highest and less variable distance of 206 rkm (SD = 40) in 2021. Construction was completed in 2023 to replace the Drayton Dam with a full river width rock-rapids style fishway. Assuming the new structure improves passage over a range of flows, it follows that annual fall migrations by Bigmouth Buffalo will continue farther upstream to exploit the fitness benefits of this now fully accessible habitat.
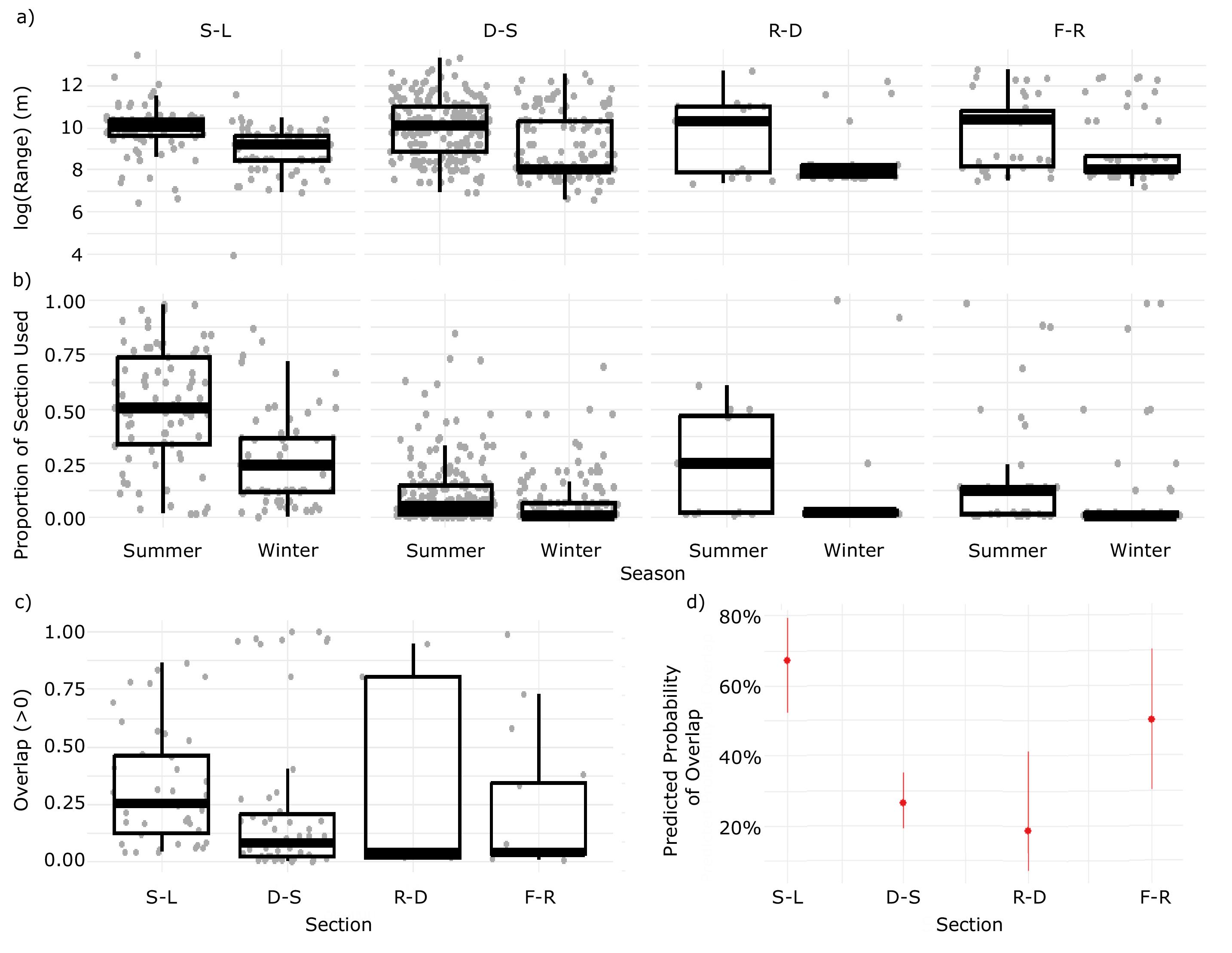
Home range size did not vary significantly between river sections; however, the proportion of river section covered by an individual's median home range decreased with section length (
Fig. 6). Despite the high degree of heterogeneity in available habitats between SALD and Lake Winnipeg (
Enders et al. 2019), summer-winter home range overlap was relatively high in this section. For Bigmouth Buffalo, there appears to be an upper bound, similar to migratory Blue Sucker (
Cycleptus elongatus) in the middle Missouri River (
Neely et al. 2009), and a lower bound for home range size. These limits can be mediated by resource availability and concurrent factors such as individual body size (
Woolnough et al. 2009), social interactions (e.g., dominance;
Bachman 1984) or predation risk (
Lima 1998) and harvest (
Marantz et al. 2016). In the Red River system, the upper and lower bounds were season specific, with winter home ranges notably smaller than observed in summer. In fact, several of the individuals were only detected at a single receiver in winter. This reduction of home range size is expected, as fish reduce metabolic rate and activity with decreasing water temperature (
McMeans et al. 2020). With additional environmental and fish detection data, such observations will be crucial for understanding the impact of climate change on migratory behaviour (
Robinson et al. 2009;
Kubelka et al. 2022;
Howard et al. 2023). For example, genomics, recruitment surveys, or fish community dynamics could help reveal the indicators and consequences of climate change and river fragmentation on Bigmouth Buffalo (
Newson et al. 2009;
Bloomfield et al. 2022;
Lackmann et al. 2023).
It is important to note that our interpretations of home range size, migration date, and rate of movement were limited by the study design. For example, error in these estimated metrics will vary from the southern to northern extent of the study system because receiver stations are separated by increasing distances (
Fig. 1). Additionally, the use of minimum linear extent as a measure of home range includes extraneous movements that can result in larger estimates of home range compared to other analytical approaches (
Vokoun 2003). Receiver detection efficiency at rkm 24 was > 80% up to 400 m in summer and winter, covering the full river width in most sections (Table S2). Nevertheless, large receiver spacing coupled with missed detections does impact how migration is observed. Other explanations for the drivers of migratory movements might emerge with the collection of additional data, such as bathymetry or through tagging more large individuals to detect and statistically control for body size effects among river sections.
Enders et al. (2019) found low transition probabilities from sections downstream to upstream of both SALD (0.005) and Drayton Dam (0.455). In the current study, we found that the reduced connectivity imposed by these dams influences seasonal home range overlap. Notably, the longest unimpeded section of river, between Drayton Dam and SALD, had the lowest probability of overlap (
Table 3) and a pronounced separation of seasonal habitats. This supports the hypothesis that adequate geographic space can lead to seasonally distinct habitat use in a heterogeneous environment. On the contrary, the highest probability of overlap occurred in the section of river between SALD and Lake Winnipeg where space was relatively limited (
Table 3). Although differential space use occurs in other riverine species (e.g., Blue Sucker, Lake Sturgeon (
Acipenser fulvescens), and Flathead Catfish (
Pylodictis olivaris),
Vokoun and Rabeni 2005;
Neely et al. 2009;
Colborne et al. 2019), the competing influence of river fragmentation is less well understood. Fish that managed to pass upstream of the structure exhibited mixed behaviours including migration, apparent straying or section residency. Variation in behaviour should be expected (
Kerr et al. 2010;
Chizinski et al. 2016;
van Leeuwen et al. 2016), particularly given that large Bigmouth Buffalo could achieve extraordinary ages and spawn periodically in the Red River (
Lackmann et al. 2023). With nearly half of those crossing SALD undergoing migration, we believe there is strong evidence to suggest much of the Bigmouth Buffalo population downstream of SALD are unable to maximize fitness similar to conspecifics upstream. In small, fragmented river sections, such as downstream of SALD and upstream of the former Drayton Dam, fish have restricted access to habitats, including those which are most seasonally profitable (
Enders et al. 2019). Rehabilitation of the Drayton and similar in-stream barriers should have positive consequences for potamodromous Bigmouth Buffalo.
Over half a decade of telemetry data has provided an unparalleled look into the migratory behaviour of Bigmouth Buffalo in a fragmented riverscape, emphasizing the critical role of river connectivity for accessing distinct summer and winter habitats. The study system was marked by notable migratory behaviours and distinct seasonal home ranges across different river sections, underscoring how anthropogenic developments such as dams can impact the distribution of migratory fish by limiting access to preferred habitats. Disruptions to habitat connectivity can restrict access to profitable habitat and impose individual fitness and population-level consequences that may take decades to manifest in long-lived species. Bigmouth Buffalo met our migration criteria only in the longest unimpeded river section, with movements generally directed downstream in spring and upstream in fall. Variability in migratory behaviour was linked to factors including flood duration and timing. The study also documented partial migration, a phenomenon critical for population stability and resilience under heterogeneous environmental conditions. These findings should be valuable to resource managers and policymakers concerned with habitat deficiencies and who plan strategic remediation in recovery actions for Bigmouth Buffalo and other migratory species.