Introduction
There is growing concern about the fate and effects of microplastics (plastics < 5 mm in size) in aquatic ecosystems due to their abundance and persistence in the environment, and their potential toxic effects on aquatic organisms (
Horton et al. 2017;
Wang et al. 2019;
Bucci et al. 2020). Plastic polymers are synthesized primarily from fossil fuels like petroleum or natural gases, and thus are carbon (C)-based products. Due to the large amount of plastic entering aquatic ecosystems, plastic-derived C could be considered a significant C pool in the environment (
Dees et al. 2021).
Slowly, macroplastics are fragmented through abiotic degradation (i.e., photo- and mechanical degradation) in surface water, leading to the generation of microplastics, which are more susceptible to further biodegradation by microbes (
Chamas et al. 2020;
Zhu et al. 2020;
Dees et al. 2021;
Priya et al. 2022). During these processes, degradation of microplastics can result in the release (termed “leaching”) of small dissolved organic carbon (DOC) compounds from microplastics into water. Further, microbial utilization of microplastics can lead to mineralization to CO
2 and CH
4 (
Curran and Strlič 2015;
Zhu et al. 2020;
Lee et al. 2021;
Priya et al. 2022). In addition to the fragmentation and mineralization of macro and microplastics in the environment, microplastics can foster microbial growth more than natural DOC, suggesting that microplastics could increase microbial activity and enhance the mineralization of microplastics in the environment (
Sheridan et al. 2022).
Previously, compound-specific isotope analysis (CSIA) revealed that in laboratory experiments, microbial degradation of microplastics resulted in mineralization to respired CO
2 and incorporation of plastic C in cell membrane components of microbes (
Taipale et al. 2019,
2023). Further, this plastic-derived C was then trophically transferred and utilized by algae and zooplankton (
Taipale et al. 2019,
2023). These studies suggest that MP-derived C can be effectively used by mixotrophic algae in the formation of biomolecules. However, the degradation and subsequent incorporation of plastic-derived C into aquatic food webs has not been studied in the natural environment and a better understanding of these processes under such conditions is needed (
Sander et al. 2019).
The objective of this experiment was to measure the leaching of C from microplastics over time in a boreal lake ecosystem and determine if we could detect the leached C within the aquatic food web. We employed isotopically labelled
13C-polystyrene (
13C-PS) as a novel approach to trace MP-derived C in an aquatic food web (
Liu et al. 2024). We added
13C-PS to a 1100 L limnocorral (in-lake enclosure, open to sediment and enclosing natural water and biota of the lake environment). Potential plastic leaching and incorporation into the food web was measured over time using stable isotope analysis of δ
13C-DIC (dissolved inorganic carbon), δ
13C-DOC, and δ
13C in amino acids (δ
13CAA) of periphyton and plankton. We hypothesized that (1) microplastics in a lake lose C over time due to both abiotic (photo-oxidative) and biotic (microbial) degradation, and (2) that DOC and DIC derived from microplastics is bioavailable and incorporated into the food web through utilization by microbes. From these hypotheses, we predicted that (1) DIC and DOC would be
13C-enriched (relative to a control; measured as δ
13C-DIC and δ
13C-DOC) following addition of
13C labelled microplastics, and (2) amino acids in plankton and periphyton would be
13C-enriched (relative to a control; measured as δ
13C in amino acids) following addition of
13C labelled microplastics.
Discussion
We investigated the degradation of microplastics under natural conditions using 13C-PS in a limnocorral experiment. The results of this small study highlight that microplastic degradation is a slow process under natural conditions, and more work is needed to understand the timescale of degradation in the environment. We found minimal evidence of microplastic degradation and C leaching as DIC, DOC, and in periphyton and plankton. The value of this work, as well as its limitations and opportunities for future research, are discussed below.
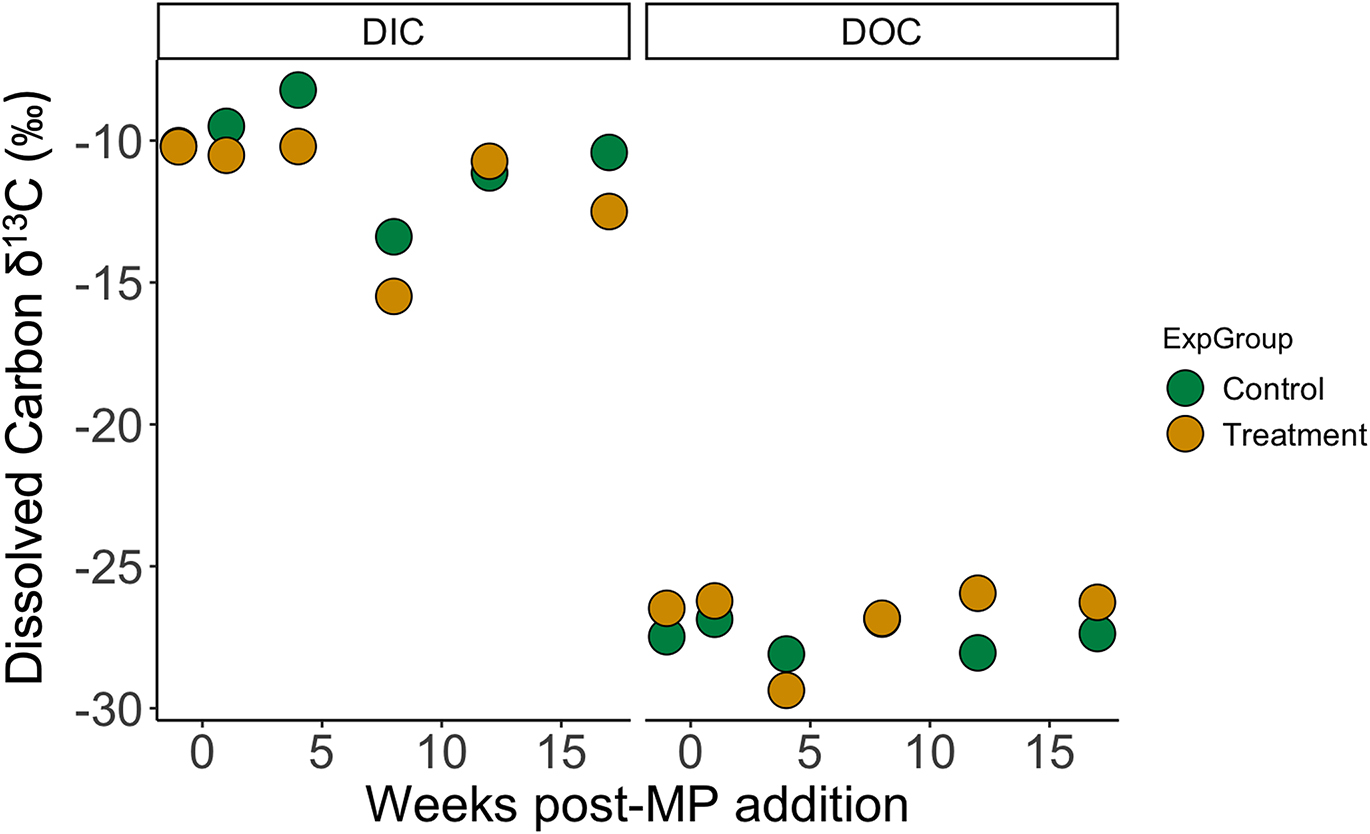
Based on the relative difference in δ
13C-DOC between the control and treatment limnocorral, we estimated that the leaching rate of plastic C herein was 0.69% year
−1. Due to this slow rate of microplastic degradation, we were unable to detect any significant DOC production using δ
13C-DOC in the present experiment outside the range of natural variability. Since this experiment was completed, two laboratory studies measuring degradation rates of
13C-labelled microplastics (polyethylene (PE) and PS) in humic and clear lake water have been conducted. In closed bottle experiments with organic C-depleted natural lake water,
13C-labelled PS was added and δ
13C was measured in gas and microbial fatty acids over 6 weeks (
Vesamaki et al. 2022). Polystyrene degraded at a rate of 0.22% year
−1 in humic lake water and 0.17% year
−1 in clear water (
Vesamaki et al. 2022). Estimated degradation rates of PE in similar bottle experiments, again conducted with humic and clear lake water, were 0.45% year
−1 in humic lakes and 0.07% year
−1 in clear lakes (
Taipale et al. 2022). Despite the use of different polymers (PE vs. PS), with different chemical and physical properties (PE is an aliphatic, positively buoyant polymer vs. PS is an aromatic, neutrally buoyant polymer), their calculated rates of degradation in humic lakes were similar to that measured herein (0.69% year
−1).
Potential differences in environmental conditions between these laboratory studies versus the field (i.e., differences in UV radiation, temperature, microbial community composition) did not appear to largely influence the degradation rate of PS, but both studies highlighted that microbial community composition will strongly influence the biodegradation rates of microplastics because certain species are capable of decomposing plastic polymers (
Taipale et al. 2022;
Vesamaki et al. 2022). In particular, species in humic lakes may be more adept at decomposing strongly recalcitrant materials like plastic, although this appeared to be more important for PE than for PS. The lake studied herein was not particularly humic (DOC = 7.27–9.15 mg/L), although we did not study microbial community composition in the limnocorrals and cannot confirm whether any known species capable of biodegrading microplastics were present.
Using the degradation rate of PS calculated by
Vesamaki et al. (2022), we re-evaluated the changes in δ
13C we would expect to see over our experimental period. At a degradation rate of 0.22% year
−1 we estimated that δ
13C-DOC herein would increase 0.4‰ relative to the control value (see calculations in Supporting Information, Part C). Indeed, we did observe a change of this magnitude for PS in the
13C-microplastic limnocorral and calculated a similar degradation rate of 0.69% year
−1 using our δ
13C-DOC data (see calculations in Supporting Information, Part D), but this rate was not statistically significant based on our experimental design. Based on the low variability in control values throughout the experiment, and these low degradation rates estimated previously, our inability to detect changes in δ
13C of DOC and DIC were likely related to low degradation rates and low mineralization of DOC and DIC in the water column rather than limited statistical power.
At the time this experiment was conducted, only one previous study, to our knowledge, had reported rates of microplastic degradation in terms of C loss. When plastic C loss and DOC production of expanded PS (EPS) was measured during a 2-month photochemical degradation experiments, total plastic C loss was 7% over the 2 months, and total DOC production was 6.8% of initial plastic C (
Zhu et al. 2020). We initially used this laboratory-derived rate of EPS degradation to estimate the amount of C that could be expected to leach from the
13C-PS following addition to the limnocorral in Lake 378. Based on their findings, we calculated that if 7% of added
13C-PS leached as DOC over 2 months, measured δ
13C-DOC in the experimental limnocorral would be 7.4‰, a value well outside the range of control δ
13C-DOC values herein and the natural range of –29 to –19‰ (see Supporting Information, Part A: Table S1 and Zigah et al. 2011). However, degradation of C from microplastics in the natural environment we investigated herein was much slower than this previous laboratory experiment would suggest, and δ
13C-DOC in our experimental limnocorral never exceeded –25.9‰, indicating that degradation of plastic could not be detected under the experimental conditions. While a slower degradation rate of PS herein could be expected given that PS has since been shown to leach C at a slower rate than EPS (
Romera-Castillo et al. 2022), comparisons of our current experiment to
Zhu et al. (2020) are largely limited because of differences in UV radiation, which often initiates the breakdown of microplastic polymers (
Emad and Raghad 2013;
Chamas et al. 2020). The present study was conducted outdoors under natural UV conditions in a lake, while
Zhu et al. (2020) irradiated microplastics under a solar simulator, which could have a substantial influence on the measured degradation rate of microplastic and result in the contrasting degradation rates observed. Another difference between our study and
Zhu et al. (2020) was the use of freshwater versus seawater, but this apparently does not have an effect on C leaching from microplastics (
Egea et al. 2024).
Our study of the incorporation of plastic-derived C into the food web is comparable to another laboratory experiment by
Taipale et al. (2023), wherein microplastic PS degradation and C incorporation was measured and compared to terrestrial leaves and lignin, similarly small amounts of
13C labelled PS were measured in zooplankton. Considerably larger amounts of
13C-PS (30 mg) were added to smaller volumes of lake water (2.6 L), but still, only small amounts of
13C-PS were detected in amino acids (up to 3%) and fatty acids (up to 2.5%;
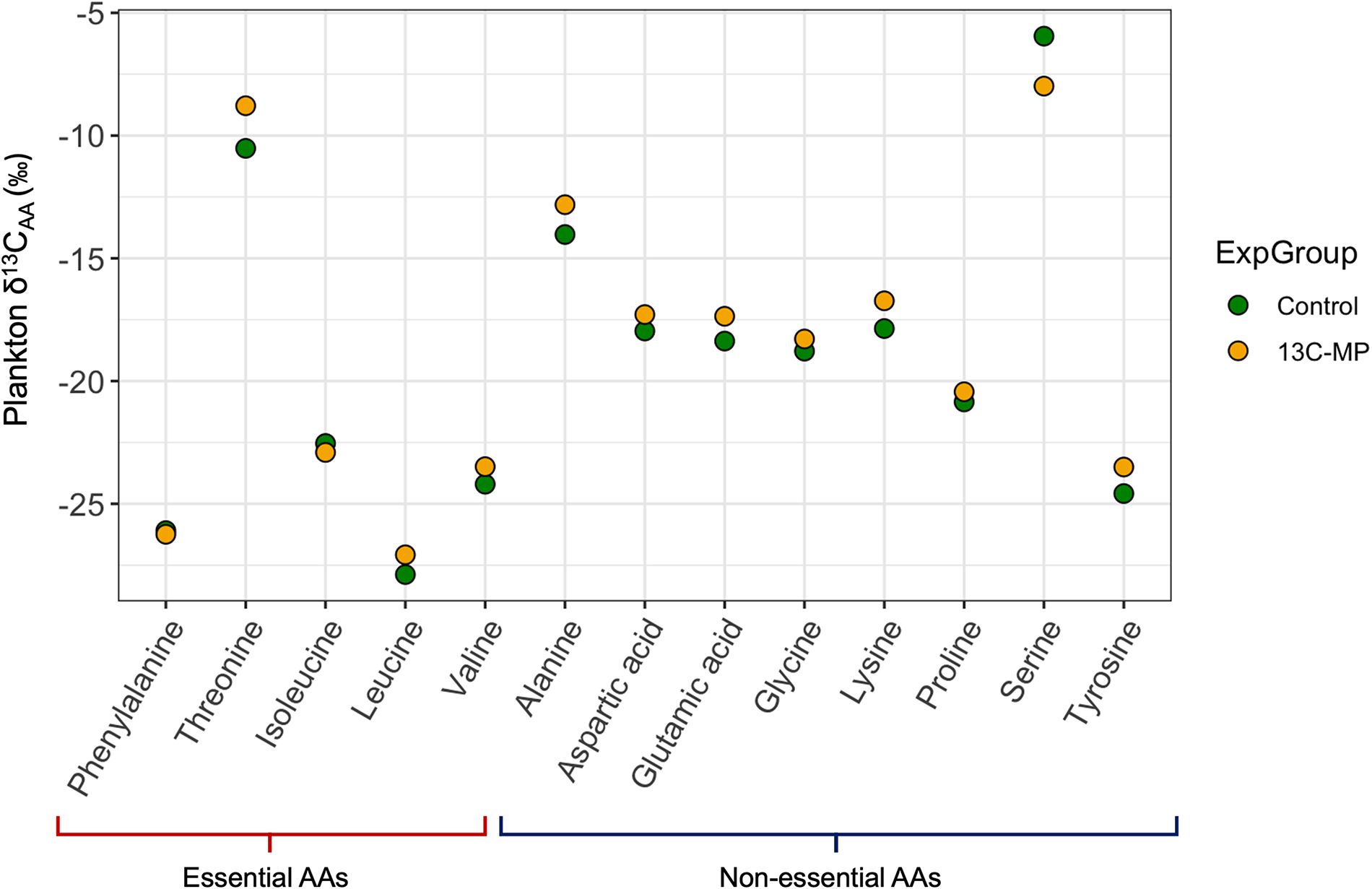
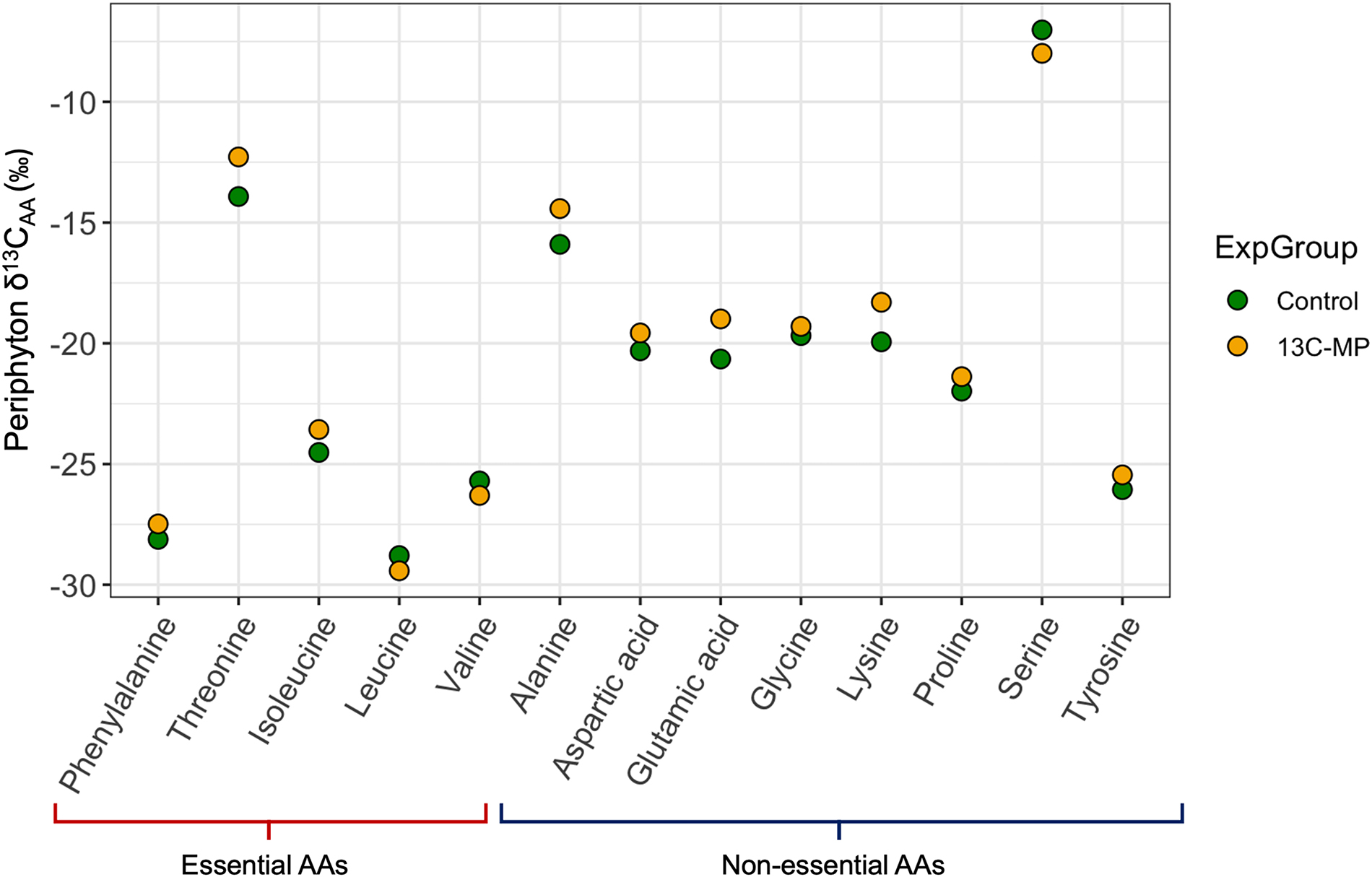
Taipale et al. 2023). Similarly, herein, most amino acids were significantly (albeit only slightly) enriched in
13C in the treatment limnocorral relative to the control (0.2–1.7‰ in 11 out of 14 AAs for plankton, 10 out of 14 AAs for periphyton). Collectively, these results may suggest evidence of degradation of microplastics at a similar rate. Unfortunately, we cannot definitively state whether our small increases in δ
13C-DOC and most of the δ
13CAA values were a result of a small amount of leached
13C-microplastic or simply natural variability in these endpoints due to limitations in the design of this pilot-scale in situ experiment.
In the present study, if we had anticipated a much slower degradation rate and added a greater amount of
13C-PS, we may have been able to detect greater
13C enrichment. For instance, we estimated based on our observed leaching rate (0.69% year
−1) we would need to add 430 mg of
13C-PS to detect δ
13C-DOC outside the range of natural variability (i.e., >–19‰). We also estimated that based on this leaching rate, in the current study it would have taken 2.9 y to detect δ
13C-DOC outside the range of natural variability (i.e., >–19‰). In addition, a longer experimental period may have helped to detect
13C enrichment; however, we ran the experiment as long as possible during the full open-water season (May–September) and were limited by destruction of the mesocosms by ice-off in the spring. Further, due to the logistical difficulty of recollecting and counting these small microplastic particles, we cannot confirm the physical fate of the microplastics. We designed the experiment anticipating that the majority of PS fragments of this size range would remain in suspension for the 4-month experiment, in agreement with residence time calculations for PS done by
Elagami et al. (2022). However, in contrast to previous literature and the known properties of virgin PS (
Driedger et al. 2015;
Rochman et al. 2019), companion studies in Lake 378 revealed that neutrally buoyant polystyrene either collected in a microlayer on the water surface or sank rapidly from water to sediment in limnocorrals in this lake (
Rochman et al. 2024; Graves et al. Pers. Comm.). Therefore, any MP-derived C would more likely be water-surface or sediment-associated than in the water column. In retrospect and in future studies, given this new information, sampling could focus on the water surface and/or sediment and pore-water to understand the fate of MP-derived C.
In combination with slow degradation rates, one possible reason that we could not detect any significant plastic-derived C in the water column is that these leached compounds are expected to be highly biolabile and may be readily assimilated or utilized by microbial communities (
Romera-Castillo et al. 2018;
Zhu et al. 2020). Further,
Taipale et al. (2019) also observed that C from plastic was more likely respired than utilized in macromolecules, suggesting that it may be more difficult to detect leached C incorporated into amino acids. Our experiment took place outdoors in the natural environment using 1 m wide in-lake enclosures that were open to the sediment and atmosphere, whereas previous studies have been conducted using bottle experiments in the laboratory (
Taipale et al. 2019,
2022;
Zhu et al. 2020). We likely encountered greater background noise and more difficulty detecting significant changes in
13C. For instance, we were not able to detect even a slight enrichment of
13C in DIC in the present experiment, suggesting that either respired DIC derived from microplastics was present only in a low/nondetectable amount, or potentially this produced gas quickly evaded to the atmosphere and was not accurately captured in our aqueous DIC samples.